Journal of Surgery and Surgical Research
Current Opinion in Clinical Management of Human Cystic Echinococcosis in the Liver
António Menezes da Silva*
General Surgeon, Co-coordinator for Surgery of the WHO-IWG on CE, Portugal
Cite this as
Da Silva AM. Current Opinion in Clinical Management of Human Cystic Echinococcosis in the Liver. J Surg Surgical Res. 2024;10(2):030-038. Available from: 10.17352/2455-2968.000164Copyright
© 2024 Da Silva AM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Cystic echinococcosis remains a serious and threatening disease in countries on all continents where it is endemic. It is a zoonosis that continues to be neglected, despite all the efforts of the teams working on it and the WHO. Therefore, it is never too much to remember the clinical management of this disease in humans. This is what we intend in this manuscript. The disease is caused by the larval stages of cestodes (tapeworms) of the genus Echinococcus found in the small intestine of carnivores, which infects humans due to accidental ingestion of eggs of these parasites. The most frequent and most widespread across all continents is cystic echinococcosis, caused by Echinococcus granulosus (sensu lato). The annual incidence of CE can range from less than 1 to 200 per 100,000 inhabitants in various endemic areas. In China and Central Asia, the population risk is more than 40 million people, with a mortality rate of about 2% - 4%.
Objectives: One of the objectives of this manuscript is to recall the parasite cycle and the form of contamination, a very important public health aspect, and draw attention to this neglected zoonosis that affects millions of people, particularly in the countries of Central Asia, the Mediterranean basin and South America. Last but not least, we intend to draw attention to the correct nomenclature in relation to the terms that define the disease, the different stages, and the proper designations, particularly with regard to therapeutic modalities.
Method: The manuscript also aims to describe the form of contamination of the disease, the diagnostic methods, in particular the use of ultrasound, and the therapeutic modalities, some of which, particularly the invasive ones, must be carried out in Centres dedicated to the study and treatment of this zoonosis.
Conclusion: Despite these important data and the socioeconomic impact Echinococcosis remains a neglected disease. Given this situation, all forms of echinococcosis remain serious public health threats, particularly in South America, the Mediterranean basin, and Central Asia, which must deserve the attention of the states of these continents and the WHO.
Introduction
Echinococcosis is the generic name for the disease caused by parasites of the genus Echinococcus, which belong to the Teniidae family. Although there are different species of Echinococcus described, only four of them – E. granulosus, E. multilocularis, E. oligarthrus, and E. vogeli – are recognized as taxonomically relevant and pathogenic for humans. To distinguish the diseases caused by those different species, the World Health Organization (WHO) proposed the designation Cystic Echinococcosis (CE) for the disease caused by E. granulosus, Alveolar Echinococcosis (AE) for the disease caused by E. multilocularis, and Neotropical echinococcosis the disease caused by Echinococcus vogeli and oligarthrus. These two species and the disease they cause are practically exclusive to the so-called "New World".
The species E. granulosus is the most frequent, with strong evidence of the existence of at least seven strains with different degrees of pathogenicity [1]. Many genotypes of E. granulosus have been identified that differ in their distribution, host range, and some morphological features; these are often grouped into separate species in modern literature. The known zoonotic genotypes within the E. granulosus sensu lato complex include the “classical” E. granulosus sensu stricto (G1–G3 genotypes), E. ortleppi (G5), and the E. canadensis group (usually considered G6, G7, G8, and G10). Research on the epidemiology and diversity of these genotypes is ongoing, and no consensus has been reached on appropriate nomenclature thus far.
Although the same strain can infest different hosts [2,3], and all of them are equally responsible for maintaining the Echinococcus life cycle, it is sheep that are the most important intermediate hosts and the respective strain is the one with the highest degree of pathogenicity for humans. Variations of the same strain can occur, however, in different endemic regions [4], this fact can alter the pathogenicity of these strains and influence the prognosis in infested patients, therefore, knowledge of the different strains, their variants, and respective hosts are important factors for the surveillance and control of CE [4-6].
Despite all the efforts of the various teams working in these countries and the WHO, Cystic Echinococcosis remains a serious, threatening, and neglected disease in countries on all continents where it is endemic [5,7].
Echinococcus life cycle
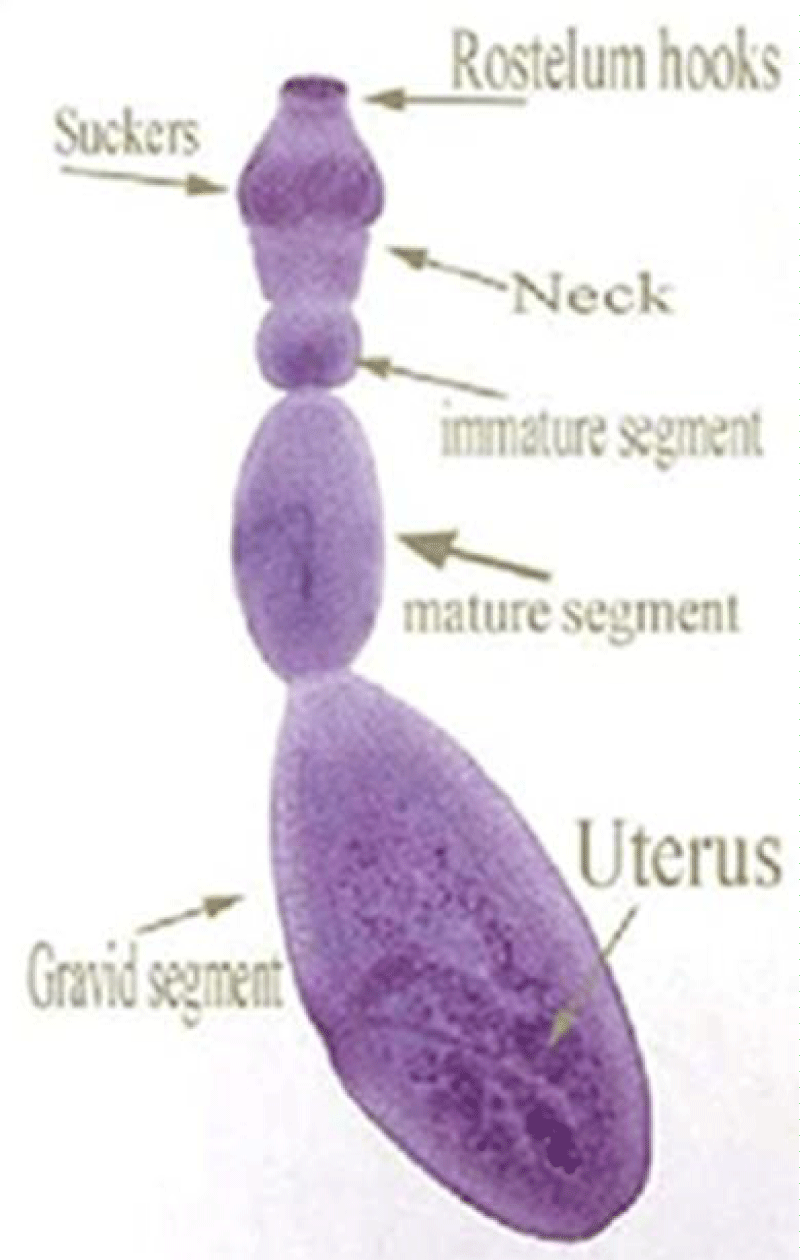
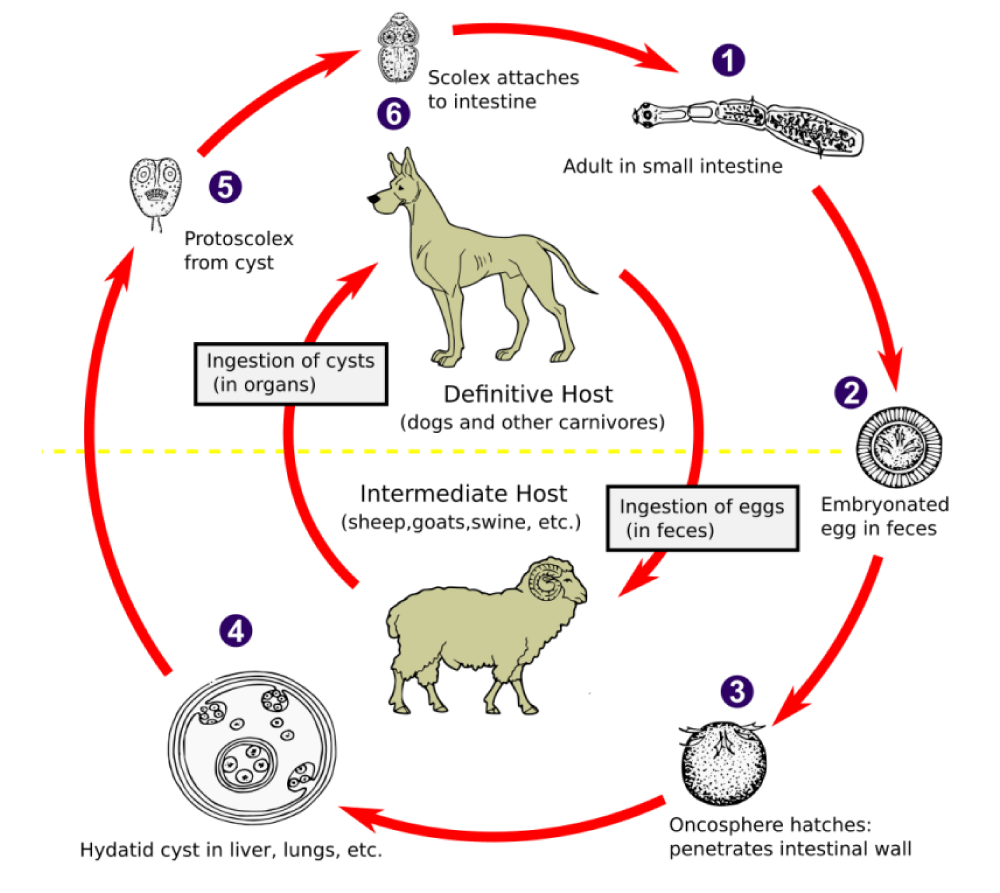
The adult form of E. granulosus tapeworm measures around 5 mm and lives in the intestine of some carnivores, predominantly dogs, called definitive or final hosts. The larval phase of the parasite's development takes place in an herbivore or, accidentally, in humans, designated as intermediate hosts. The parasite has 3 parts (Figure 1): the head, with hooks and suction cups; the neck; and the body, which has 3 segments with only the last being ovigerous, with hundreds of eggs. The eggs are eliminated with the carnivore’s feces and thus dispersed in pastures or near water courses, and can be ingested by herbivores – intermediate hosts – in which the larval stage of the parasite occurs. Humans, as mentioned, are accidental intermediate hosts, becoming contaminated in one of two ways: directly, through contact with an infested dog; or indirectly, through water or poorly washed raw foods food contaminated with feces from infested dogs.
The eggs, with a diameter between 40 and 50 μm, after being ingested by the intermediate host, reach the stomach where the hexacanth embryo is released. The embryo passes to the intestine and, crossing its wall, reaches the portal system and reaches the liver, where it can attach and develop, or, it surpasses the hepatic barrier and goes to any other organ. Precisely the same happens in humans. After attaching itself to an organ, the hexacanth embryo slowly transforms into a spherical formation in some organs which, due to its similarity to a cyst, has been called a hydatid cyst.
If these cysts rupture, their contents will spread and the liberated protoscolices may create secondary cysts [8] in other sites within the body giving rise to secondary echinococcosis. The definitive host becomes infected by ingesting the cyst-containing organs of the infected intermediate host. After ingestion, the protoscolices evaginate, attach to the intestinal mucosa, and develop into adult stages in 32 to 80 days, closing the parasite's life cycle (Figure 2). The growth of a hydatid cyst is directly related to the progressive increase in the fluid contained within it. Throughout its slow evolution, several situations may occur: death of the parasite due to germinal layer function loss, due to its aging and detachment; the transformation of scolices into daughter vesicles making the cysts multivesicular, in an attempt to preserve the species; the cracking of the cyst wall, by detachment of the membranes or by, microtrauma.
The protoscolices ensure the fertility of the cyst. They are resistant to refrigeration and can survive for 10 days in putrefying carcasses, characteristics that have important consequences for the spread of the disease [5].
Throughout the evolution of the hydatid, some protoscolices can give rise to daughter vesicles (vesiculation), which float in the hydatid fluid, and have a constitution and reproductive role identical to that of the mother vesicle [7,9,10]. Protoscolices, therefore, have a double potential: to give rise to an adult form of the parasite and to produce new cysts.
Historical facts of the disease
The hydatid cyst, the lesion in intermediate hosts corresponding to the larval stage of the parasite, has been known to man for thousands of years. It was first described by Hippocrates as “vesicles filled with water” (Adams, 1946). Aretaeus and Galen, in the 1st century, also referred to it, and later, in the year 900, Rhazes, in his book “Al-Hawi in Medicine”, described a hydatid cyst of the liver, which he had found in several slaughtered animals, comparing it to a water balloon (Thomas, 1884).
Only in the 17th century was recognized, by Francesco Redi (1664) the animal origin of the hydatid cyst, and only a few years later P. J. Hartmann (1683) attributed its parasitic nature, later confirmed by Edward Tyson in 1687 (Dew, 1928). The first studies of the parasite were due to Pallas (1776), Goeze (1782), and Batsch (1786): the first to recognize hydatid cysts as living organisms and observe the teniform characteristics of the protoscolices. Goeze gave a detailed description of the suckers and hooks of the scolex, which he named Tenia visceralis socialis granulosa, and Batsch observed under the microscope and described the larval form of the parasite with its crown of hooks.
In 1808, Rudolphi studied the adult form of the parasite in the intestines of dogs, giving it the name Echinococcic, which gave rise to the current name Echinococcus. The first case of a hydatid cyst in man was observed by Low, in North America, in 1808, although the first report was due to Bremser, in 1821 (Romero-Torres and Campbell, 1965), as that would only come to be communicated in 1822, in Lyon. In 1853, Carl von Siebold definitively established the relationship between the adult form and the larval form of the parasite, after experimental studies in which he parasitized dogs with hydatid cysts from sheep, marking the beginning of modern helminthology. Thomas (1855) in Australia, Naunyn (1863) in Berlin and Krabbe (1866) in Iceland, demonstrated, with identical studies, the participation of man in the parasite cycle.
Pozzi, a French surgeon from Lourcine-Pascal hospital in Paris, performed the first cystectomy in 1887 which, after the work of Pothrat and Baraduc in 1895, became the preferred treatment method for Russian surgeons, with Napalkoff standing out who performed it without opening the cyst, and it is still the ideal procedure today when the cyst is completely removed.
In 1901, Felix Dévé [8], from Rouen, demonstrated, for the first time, the vesicular evolution of scolices. He is also responsible for the definition of a hydatid cyst, made up of two distinct parts – the parasite and the adventitia that surrounds it. The adventitia is a layer of inert tissue of variable thickness with fibrosis, which results from the host’s organ reaction against the hydatid, which is a foreign body.
Also at the beginning of the last century, two serological diagnostic techniques emerged for the diagnosis of CE – the complement fixation reaction, known as the Ghedini-Weinberg reaction, developed between 1906 and 1909 (Weinberg, 1909), and the Casoni intradermal reaction, described in 1911 – which have been used for decades to diagnose human CE.
In 1923, Mazzaocco studied the composition of hydatid fluid, finding characteristics identical to those of the host's serum, notably in the concentrations of sodium, potassium, chlorine, and carbon dioxide.
Only in the 1960s, did new diagnostic techniques emerge, such as latex agglutination, indirect hemagglutination, and indirect immunofluorescence. In 1967, the Lille Parasitological School published a study on the antigenic structure of Echinococcus granulosus, describing a specific fraction, defined by immuno-electrophoresis, initiating a new stage in the diagnosis of CE.
With the advancement of technological imaging means, ultrasound emerged with a prominent role in the diagnosis of CE. Gharbi [11] and collaborators proposed, in 1981, a classification for the hydatid cysts, based on the different evolutionary stages of the parasite, obtained by ultrasound images, defining five types. Later, other authors presented new classification proposals (Beggs, 1983; Lewall and Mccorkell, 1985, Caremani, et al. 1991, Perdomo, 1995, Shambesh, et al. 1999), with several types and subtypes, corresponding, in essence, to modifications of the classification proposed by Gharbi. In order to achieve uniformity of criteria in this ultrasound classification, the WHO created, in 1995, a Working Group led by Callum Macpherson, for its standardization. After multiple discussions and meetings, the ultrasound classification ended up being approved in 2001 [12].
In relation to the treatment of hydatid cysts of the liver, Jean Demirleau stood out in that decade, publishing in 1973, the book “Traitement des kystes hydatiques du foie”, in which he defended a different therapeutic approach for univesicular and multivesicular cysts, for considering the difference between them significant. Another surgeon – Farrokh Saidi – published, in 1976, the book “Surgery of Cystic Echinococcosis ” which addresses the entire topic of Cystic Echinococcosis, calling into question the effectiveness of scolicidal agents in multivesicular cysts and drawing attention to the risks of using these products in cysts communicating with the bile ducts.
In 1974, Heath, et al. [13] demonstrated the effectiveness of benzimidazole compounds against E. granulosus, which led to the use of these drugs in the therapy of CE and, in 1977, Bekhti, et al. published a study in which they reported the success of four cases treated with Mebendazole. Other authors followed his experience and published the results of therapy with Albendazole (Verheyen in 1982, Saimot et al and Morris, et al. in 1983, Horton, et al. in 1989, Gil-Grande, et al. in 1993, and Teggi, et al. in 1999).
Still in the 1970s, ultrasound began to be used to detect lesions caused by parasitic infestations, including hydatid cysts (WHO-IWGE, 2003), proving to be the ideal means of diagnosis, particularly in screenings in rural areas, due to the possibility of using portable ultrasound.
In 1981, the World Health Organization (WHO) published a new manual disclosing several immunoprecipitation techniques applied to CE: the arc 5 Double Diffusion Test (DD5), the indirect Immunofluorescence Test (IFA), and immunoenzymatic methods, known by the acronym ELISA (Enzyme-linked immunosorbent assay), in which specific antibodies are highlighted using a soluble antigen and using an antiserum marked by an enzyme.
In 1983, Fornage reported an accidental puncture of a hydatid cyst of the liver without verifying the complications that for years made this gesture prohibited. That same year, Ben Amor treated hydatid cysts of the liver in sheep by aspirating the fluid from the cyst and injecting hypertonic sodium chloride into the cystic cavity. The good result obtained encouraged the application of this procedure in two patients with absolute contraindication to undergo general anaesthesia, with a result that was considered excellent, although the first case of successful percutaneous drainage, performed on a recurrent hydatid cyst of the liver, was published by Mueller, in 1985.
In the same year, Livraghi carried out, without complications, the percutaneous aspiration under ultrasound control of two hepatic cysts and, in 1987, Filice, et al. began the treatment of hydatid cysts by percutaneous drainage, using alcohol 95% as a scholicide.
These reports and the acceptance of the mini-invasive treatment method radically changed the treatment of CE, which led to the WHO setting up a Working Group at the end of 1996, to evaluate the possibility of applying this new treatment method, which came to be known as PAIR, published in the WHO Report in 1998.
Clinical management
Diagnostic methods: In the past, the diagnosis was based on serological tests. The biological diagnosis was carried out using direct parasitological methods and immunological methods, these being the most accurate. Immunological tests make it possible to demonstrate the host's immune response to the infestation, identifying anti-Echinococcus antibodies or researching circulating antigens. However, despite the development of specific techniques, the immunological diagnosis of hydatid cysts is still a difficult task, with a high percentage of false negatives.
Adjunctive methods, such as the detection of serum antibodies against the parasite [14-16], can aid in moving closer to a definite diagnosis. Serological assessment typically follows a two-step approach: in the first step, diagnostically sensitive tests are employed [e.g., indirect haemagglutination test, IHA, or enzyme-linked immunosorbent assay (ELISA)] using E. granulosus crude antigens. However, these tests lack specificity and cross-react with other helminthic infections and also gastrointestinal malignancies; a second step, a highly specific test, is used to confirm the results of the screening tests.
Many factors may influence test results [17], including the age and stage of the cyst, whether or not the cyst is intact, and whether or not extrahepatic cysts are present. Local infection pressure may influence test results, with children in highly endemic areas found to be seropositive in the absence of detectable cysts.
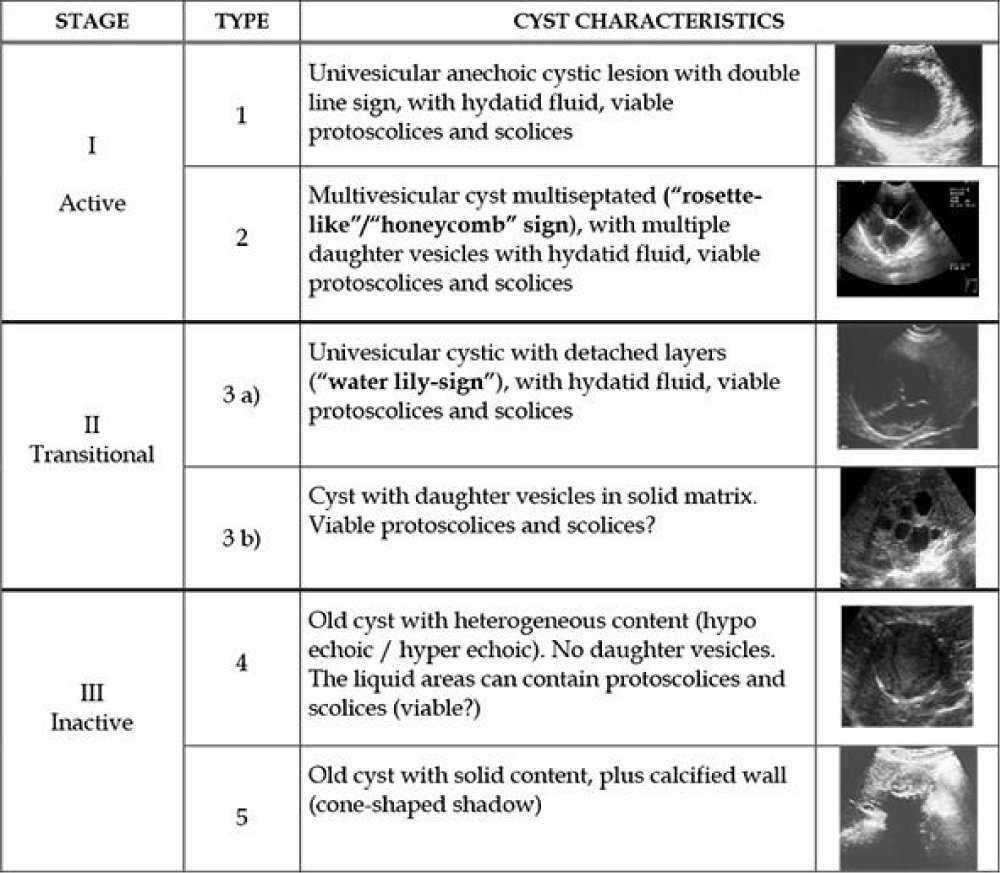
Ultrasonography is currently the most commonly used imaging method in the diagnosis of CE. In addition to the ease of execution, lower risks for patients, and lower costs, it has an important role as a tracking method in endemic areas, with the possibility of using portable ultrasounds in nomadic and in regions of weak resources. Ultrasound images are pathognomonic (Table 1) for CE in two situations: when a membrane is observed floating in the liquid contents of the cyst – the “lily pad” or “snake” sign (Sabih, et al. 1996), which corresponds to detachment of the hydatid membranes; and when there are septa inside the cyst, resembling the “wheel of a car” or a “honeycomb”, translating the presence of daughter vesicles.
In addition to showing pathognomonic signs, ultrasound allows, based on these signs, to establish a classification [11,12,18,19] of cysts that guides the choice of the treatment method.
Computed tomography (CT scan) is not a first-line examination for the diagnosis of CE, as its images are identical to those of ultrasound, especially when the cysts have pathognomonic signs [10,18], but it can be useful and necessary, as it allows us to show, better than ultrasound, the size and number of cysts and, above all, their relationships with the intrahepatic structures, an essential element for the surgical strategy. On the other hand, it is of great importance in situations of complicated cysts, namely rupture into the peritoneal cavity.
Magnetic Resonance Imaging (MRI) is the most recent imaging method and although it is not first-line for diagnosing CE, it can be a resource for clarifying doubtful cases [20,21]. On the other hand, the capacity of the method allows the study of the biliary tree without the use of contrast – magnetic resonance cholangiography – which is particularly important in cases where there is suspicion of communication between the cyst and the bile ducts. The images, in cases where there are pathognomonic signs, are also similar to those obtained by ultrasound.
Therapeutic modalities
There is no “best” treatment option for CE and no clinical trial has compared all the different treatment modalities, including “Watch and Wait”. For many years, surgery has been the only treatment available. With alternative treatment modalities advancing it is pertinent to clarify the current position of surgery among other options.
The aim of the treatment of CE is the death of the parasite and consequently the cure of the disease [7,9,10,22-24]. This objective must be achieved with minimal risks and maximum comfort for the patient, and always taking into account the prevention of complications, especially secondary echinococcosis, and relapses, particularly with methods that involve handling the contents of the cyst.
It is important that everyone working in this area uses correct terminology and that everyone understands it [9,23]. In this context, the "International consensus on the terminology to be used in the context of echinococcoses" was published in Parasite, a fundamental document to achieve this objective [25].
The means of achieving parasite death involve sterilization of the cyst contents using scolicidal or more generally anti-Echinococcus drugs, or removal of the hydatid, either by aspiration or following excision of the cyst.
In patients with complicated cysts (rupture, cyst-biliary fistula, compression of vital organs and vessels, haemorrhage) surgery maintains its place as the treatment of choice. In uncomplicated cysts, surgery is increasingly being replaced by other treatment options (percutaneous treatment, medical treatment, and watch & wait modality). The choice of the therapeutic method shall take into consideration the cysts' characteristics based on the US classification (Table 1). We can consider two options: non-invasive and invasive approaches.
Non-invasive approach
The non-invasive treatment corresponds solely to the oral administration of anti-infective drugs, also called “Medical” treatment. This term refers to the use of oral medication against echinococcus that destroys the parasite [19,26,27]. Today the correct name is anti-infective treatment.
Proven the effectiveness of benzimidazole compounds against E. granulosus by Heath, et al. in 1974 [13], these drugs began to be used in the treatment of CE, initially Mebendazole (MBZ) and later Albendazole (ABZ), either alone or associated with other methods. These drugs have larvicidal, ovicidal, and vermicidal activity, interfering with the formation of tubulin in the parasite cell, inducing the blockage of glucose absorption, producing glycogen depletion and degenerative changes in the endoplasmic reticulum and mitochondria of the germinal membrane, increasing lysosomes and producing cellular autolysis. Also Praziquantel (PZQ), after proving its effectiveness in animals, began to be used in humans. Although PZQ is often used to prevent secondary echinococcosis, prospective studies are needed to determine the effectiveness of the drug.
ABZ is currently the drug of choice [10,18,26,27-31] with its main metabolite, albendazole sulfoxide, being the effective active fraction, with a half-life of 8 and a half hours. It is administered orally, at a dose of 10 to 15 mg/kg/day, divided into two doses. The scolicidal effect appears to be dependent on the evolutionary stage of the cyst and the integrity of the germinative layer, with the scolicidal more effective in young, type 1 cysts, and less effective in type 2 cysts where failure can be greater than 50%.
ABZ is also used as an adjuvant both in patients undergoing puncture/aspiration and in patients undergoing cyst resection, to reduce intracystic tension and prevent secondary echinococcosis. Surgeons tend to administer ABZ from one week to one day before and from one to three months after intervention. The duration of treatment is dependent on surgical factors such as whether or not the cyst is opened. ABZ treatment is typically administered for one month after surgery in patients who have successfully undergone complete surgical resection of the cyst (radical procedure) or PAIR. The recommended treatment time extends to 3e6 months in patients with incompletely resected cysts (non-radical procedures), or when spillage has occurred during surgery or PAIR.
Invasive approaches
The invasive approaches can be carried out using two different methods: cyst puncture for sterilization of the cyst content [7,10,18,32-35]; and surgery [7,9,10,24,36]. Surgery was the only form of treatment for CE for centuries and remains the only radical method today. However, other approaches have emerged, both with regard to surgery and new methods of approach. The big difference between these two approaches is that sterilization is always a non-radical procedure, while surgery is the only radical method of treatment.
Non-radical procedures
In non-radical procedures, we have two options: the cyst sterilization with or without removal of the parasite (hydatid), and partial removal of the cyst.
Sterilization of cyst contents
The cyst sterilization is based on the degeneration of the hydatid layers and destruction of the elements of the hydatid fluid due to the effect of anti-parasite drugs orally taken or injected into the cyst, called also “scolicidal” if they are able to kill protoscolices [34,37-39].
Injecting a scolicide solution into the cyst cavity is the oldest method of treating liver cysts. It was considered the best method for the treatment of simple cysts, called univesicular, which correspond to type 1 in the current ultrasound classification. In the past, this procedure was performed only by open surgery, but nowadays we have two more approaches: laparoscopy and percutaneous puncture.
Percutaneous puncture is known as PAIR (Puncture, Aspiration, Injection (of the scolicide) and Re-aspiration). It is preferred and considered the gold standard for cysts type 1 and 3 since it is a minimally invasive technique, it is less painful for the patient as well and it has less complication rate, less expensive, with earlier discharge and activity resumption.
More recently, new mini-invasive techniques, called catheterization, have been described and developed. They are similar to PAIR, except for the last step: instead of full aspiration, an evacuation of the cyst content is done (PEvac) proposed by Schipper, et al. in 2002 [37], or a catheter is left inside the cyst as proposed by Okan to be used to treat cysts of any diameter. However, it is the preferential treatment for cysts larger than 10 cm in diameter and/or with a liquid content greater than 1000 mL. The introduction of a catheter allows draining of cyst fluid, with the catheter removed at the conclusion of the procedure.
Later, in 2007, Okan, with an identical procedure, proposed the destruction of the contents of the cyst, which he called the Modified Catheterization Technique (MoCat) [32]. This procedure results in the removal of the parasitic membranes in addition to the cyst’s content.
The success of percutaneous treatment is defined, by imaging, as a reduction in the cyst’s size and volume, thickening of the cyst’s wall, and solidification of the remaining structure. Furthermore, the procedure is considered to be successful if the cyst is no longer viable and complications, such as abscess formation, have not occurred.
To remove the parasite there are two different ways: the aspiration of the cyst content (the parasite or hydatid); or the excision of part of the cyst, which necessarily removes the parasite. In this case, a partial or a subtotal cystectomy is performed, which is a non-radical procedure.
Partial removal of the cyst
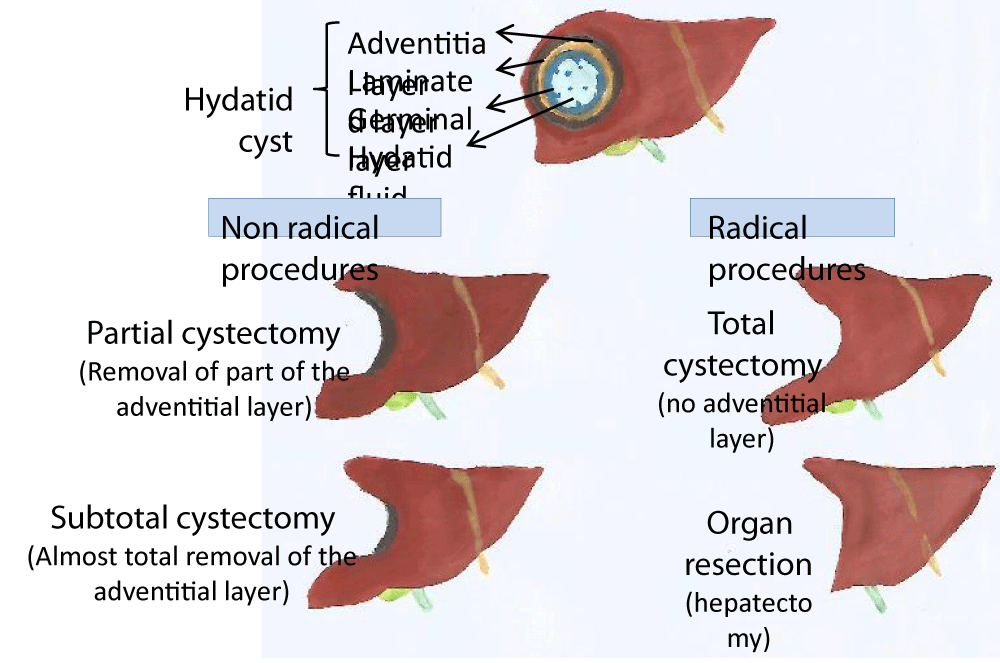
The other non-radical invasive method corresponds to a partial resection of the cyst [36,40]. This surgical method, which is widely practiced, should only be considered in situations where it is not possible to completely remove the cyst, as it may be the cause of secondary echinococcosis. This procedure only can be performed by surgery, which ideally tries to remove the entire cyst (radical treatment). However, this may not be possible, because of the risk of damage to bilious or vascular structures, so the most prudent decision will be to carry out a partial or subtotal cystectomy (Figure 3).
In this case, the cyst is not completely resected but as its contents are removed, the parasite is eliminated. However, the opening of the cyst promotes the possibility of contamination of neighbouring structures, which could lead to secondary echinococcosis. Another risk factor is infection of the residual cavity, which is left in place precisely because a total cystectomy is not performed. Simple drainage with suction and filling with epiploon (omentoplasty) are options to reduce the risk of complications.
Radical procedures (complete removal of the parasitic tissue)
The unique radical procedure corresponds to total cystectomy or organ resection (Figure 3). Total cystectomy consists of the excision of the entire cyst, which is the ideal surgical procedure, once diminishing relapses and complications. It can be performed through laparotomy or laparoscopy, both by open or closed methods. In both options, the dissection is made on the outside of the adventitia [1].
The open method is performed by opening the cyst then its aspiration and finally the removal of its content [10,24]. Only after this step, we proceed to the entire cyst “wall” removal. The closed method, known as Napalkoff’s operation, consists of the entire cyst removal without opening it. In the past the dissection was made in sane hepatic tissue (pericystectomy), to guarantee the complete cyst removal: the parasite and the host tissue that surrounds it (adventitia).
Peng Xin Yu, et al. have recently developed the periadventitial technique [1] in which the dissection is performed in the virtual space between the adventitia and sane hepatic tissue, which is a less bloody space, so it is associated with fewer complications while being as effective to remove all parasitic tissue.
Cystectomy can be performed by video-assisted surgery on selected cases [10,39], namely small cysts (5 cm in diameter) with peripheral localization. Since the total cystectomy is the ideal approach it should be done by the closed method and without using CO2 due to dissemination risk in case of rupture.
When a total resection can damage bilious or vascular structures a partial cystectomy must be done, despite this is a non-radical procedure.
Another option is hepatic resection (segmentectomy or lobectomy), in case of great-size cysts in which there is a high risk of ischemia for the remained hepatic tissue. So, hepatic resection is an option only for selected cases.
Both methods have higher risks per-operatively but fewer rates of complications and relapses after surgery. On the other side, non-radical methods have fewer intra-operative risks but a higher rate of long-outcome complications and relapses. The main advantage of radical procedures is the immediate healing of the disease which is obtained only when the cyst is completely removed whether with or without hepatic tissue (cystectomy or hepatic resection).
Although nowadays the morbidity and mortality of CE surgery have diminished, they cannot be overlooked. The prevention of complications starts with an accurate surgical technique and the necessary caution in the removal of the cysts which are very close to the bilious and vascular intra-hepatic structures. To prevent the relapses it is very important to protect the surgical field with pads soaked with scolicidal solution. This procedure will also prevent a secondary CE in case of spillage of the cyst content during the cyst removal.
Watch-and-wait strategy
This is not a therapeutic modality but rather a method of approaching cysts that, according to ultrasound images, do not appear to be active. In selected cases, CE treatment can be put on hold. This is an option for uncomplicated inactive cysts, including types 4 and 5 [41]. These cyst types appear to either remain stable in size or degenerate over time and do not compromise organ functions or cause patient discomfort. Also in small cysts type 1 this method can be suitable, however, these should undergo long-term US follow-up for at least 10 years.
It involves observing and waiting for the evolution of inactive or even type 1 cysts to understand their evolution. In any case, periodic monitoring of the evolution with imaging of the cysts is absolutely necessary to decide the need for treatment.
Conclusion
Although the parasite's cycle, its way of transmission and the means necessary to fight it are well known, the truth is that the disease persists in different countries on all continents. For these reasons, it has been included by the WHO in the Neglected Zoonotic Diseases (NZD). The decrease in the incidence of the old endemic zoonoses that had been controlled in Europe and the United States, marked a tendency to be forgotten, devoting more attention to emerging zoonoses with potential risk of pandemic, such as the case of bird flu, for example. It is clear that endemic zoonoses have common characteristics, different from emerging zoonoses, which can be summarized as: they receive little attention; they cause significant morbidity and mortality; they impose a double burden, on humans and animals; they are old diseases, eliminated in rich (developed) countries; their control, although possible, is very expensive; they do not pose a risk of global spread; they affect marginalized peoples and poor populations. This highlights the need for a global, continental, or regional task force capable of stimulating research and promoting the development of tools that favour the exchange of available data from countries where the disease is endemic. This will allow knowledge of the programmes in different regions and a flow of information between the different research teams, in order to achieve a sustainable control strategy.
- Peng X, Zhang S, Niu JH. Total subadventitial cystectomy for the treatment of 30 patients with hepatic hydatid cysts. Chin J Gen Surg. 2002;17:529–30. Available from: https://pesquisa.bvsalud.org/portal/resource/pt/wpr-519632
- Dubinsky P, Stefancikova A, Turcekova L, Macko J, Solty J. Development and morphological variability of Echinococcus granulosus. Parasitol Res. 1998;84:221-229. Available from: https://link.springer.com/article/10.1007/s004360050386
- Thompson A, Lymbery A. Echinococcus: biology and strain variation. Int J Parasitol. 1990;20:457-470. Available from: https://doi.org/10.1016/0020-7519(90)90193-Q
- Smego RA, Bhatti S, Khaliq AA, Beg MA. Percutaneous aspiration-injection-reaspiration drainage plus albendazole or mebendazole for hepatic cystic echinococcosis: a meta-analysis. Clin Infect Dis. 2003;37(8):1073-1083. Available from: https://doi.org/10.1086/378275
- Craig P, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, Gavidia CM, Gilman RH, et al. Prevention and control of cystic echinococcosis. Lancet Infect Dis. 2007;7(6):385-394. Available from: https://doi.org/10.1016/S1473-3099(07)70134-2
- Erzurumlu K, Hokelek M, Gonlusen L, Tas K, Amanvermez R. The effect of albendazole on the prevention of secondary hydatidosis. Hepatogastroenterology. 2000;47:247-250. Available from: https://europepmc.org/article/med/10690616
- Da Silva AM. Human echinococcosis: a neglected disease. Gastroenterol Res Pract. 2010;2010:1-2. Available from: https://doi.org/10.1155/2010/583297
- Dévé F. L’Echinococcose secondaire. Masson & Cie; 1946;241. Available from: https://www.cabidigitallibrary.org/doi/full/10.5555/19460800637
- Da Silva AM. Cystic echinococcosis in the liver: nomenclature and surgical procedures. J Surg Surgical Res. 2015;1(3):059-065. Available from: http://dx.doi.org/10.17352/2455-2968.000015
- Junghanss T, da Silva A, Horton J, Chiodini P, Brunetti E. Clinical management of cystic echinococcosis: state of the art, problems and perspectives. Am J Trop Med Hyg. 2008;79(3):301–311. Available from: https://pubmed.ncbi.nlm.nih.gov/18784219/
- Gharbi HA, Hassine W, Brauner MW, Dupuch K. Ultrasound examination of the hydatid liver. Radiology. 1981;139(2):459-463. Available from: https://doi.org/10.1148/radiology.139.2.7220891
- WHO Informal Working Group on Echinococcosis. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003;85:253–261. Available from: https://doi.org/10.1016/S0001-706X(02)00223-1
- Heath D, Chevis R. Mebendazole and hydatid cysts. Lancet. 1974;II:218-219. Available from: https://www.cabidigitallibrary.org/doi/full/10.5555/19750816572
- Hogea MO, Ciomaga BF, Muntean MM, Muntean AA, Mircea IP, Popa GL. Cystic echinococcosis in the early 2020s: a review. Trop Med Infect Dis. 2024 Feb;9(2):36. Available from: https://doi.org/10.3390/tropicalmed9020036
- Pawlowski Z, Eckert J, Vuitton DA, Ammann RW, Kern P, Craig PS, et al. Echinococcosis in humans: clinical aspects, diagnosis, and treatment. In: Eckert J, Gemmel MA, Meslin F-X, Pawlowski ZS, eds. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Paris: World Health Organization and World Organization for Animal Health; 2001:20–66. Available from: Available from: https://www.cabidigitallibrary.org/doi/full/10.5555/20013100616.
- Tamarozzi F, Silva R, Fittipaldo VA, Buonfrate D, Gottstein B, Siles-Lucas M. Serology for the diagnosis of human hepatic cystic echinococcosis and its relation with cyst staging: a systematic review of the literature with meta-analysis. PLoS Negl Trop Dis. 2021;15. Available from: https://doi.org/10.1371/journal.pntd.0009370
- Thompson RCA. The molecular epidemiology of Echinococcus infections. Pathogens. 2020;9:453. Available from: https://doi.org/10.3390/pathogens9060453
- Brunetti E, Junghanss T. Update on cystic echinococcosis. Curr Opin Infect Dis. 2009;22:497-502. Available from: https://journals.lww.com/co-infectiousdiseases/abstract/2009/10000/update_on_cystic_hydatid_disease.13.aspx
- WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003 Feb;85(2):253-61. doi: 10.1016/s0001-706x(02)00223-1. PMID: 12606104. Available from: https://doi.org/10.1016/s0001-706x(02)00223-1
- Lao X, Hu D, Ji L, Zhan T, Li T, Luo S, et al. Magnetic resonance imaging and next-generation sequencing for the diagnosis of cystic echinococcosis in the intradural spine: a case report. J Med Case Rep. 2023;17:466. Available from: https://link.springer.com/article/10.1186/s13256-023-04197-1
- Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, McManus DP. Echinococcosis: advances in the 21st century. Clin Microbiol Rev. 2019;32. Available from: https://journals.asm.org/doi/full/10.1128/cmr.00075-18
- Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114(1):1-16. Available from: https://doi.org/10.1016/j.actatropica.2009.11.001
- Da Silva AM. Liver cystic echinococcosis: once again the terminology. Int J Echinococcosis. 2022;1(1):19-25. Available from: https://www.bibliomed.org/gotodoi.php?mno=98434&gdoi=10.5455/IJE.2021.11.1151
- Menezes-Silva A. Hydatid cyst of the liver – criteria for the selection of appropriate treatment. Acta Trop. 2003;85:237-242. Available from: https://doi.org/10.1016/S0001-706X(02)00271-1
- Vuitton DA, McManus DP, Rogan MT, Romig T, Gottstein B, Naidich A, et al. World Association of Echinococcosis. International consensus on terminology to be used in the field of echinococcoses. Parasite. 2020;27:41. Available from: https://doi.org/10.1051/parasite/2020024
- Franchi C, Di Vici B, Teggi A. Long-term evaluation of patients with hydatidosis treated with benzimidazole carbamates. Clin Infect Dis. 1999;29:304-309. Available from: https://doi.org/10.1086/520205
- Garcia J, Bolas F, Torrado J. Bioavailability and efficacy characteristics of two different oral liquid formulations of albendazole. Int J Pharm. 2003;250:351-358. Available from: https://doi.org/10.1016/S0378-5173(02)00559-8
- Chai J, Menghebat, Wei J, Deyu S, Bin L, Jincao S, et al. Observations on clinical efficacy of albendazole emulsion in 264 cases of hepatic cystic echinococcosis. Parasitol Int. 2004;53(1):3-10. Available from: https://doi.org/10.1016/j.parint.2003.09.015
- Kern P. Echinococcus granulosus infection: clinical presentation, medical treatment and outcome. Langenbecks Arch Surg. 2003;388:413–420. Available from: https://link.springer.com/article/10.1007/s00423-003-0418-y
- Keshmiri M, Baharvahdat H, Fattahi SH, Davachi B, Dabiri RH, Baradaran H, et al. Albendazole versus placebo in treatment of echinococcosis. Trans R Soc Trop Med Hyg. 2001;95(2):190-194. Available from: https://doi.org/10.1016/S0035-9203(01)90162-2
- Rigter I, Schipper H, Koopmans R, Van Kan H, Frijlink H, Kager P, et al. Relative bioavailability of three newly developed albendazole formulations: a randomized crossover study with healthy volunteers. Antimicrob Agents Chemother. 2004;48:1051-1054. Available from: https://doi.org/10.1128/aac.48.3.1051-1054.2004
- Akhan O, Salik AE, Ciftci T, Akinci D, Islim F, Akpinar B. Comparison of Long-Term Results of Percutaneous Treatment Techniques for Hepatic Cystic Echinococcosis Types 2 and 3b. AJR Am J Roentgenol. 2017;208(4):878-884. Available from: https://doi.org/10.2214/ajr.16.16131
- Brunetti E, Troia G, Garlaschelli AL, Gulizia R, Filice C. Twenty years of percutaneous treatments for cystic echinococcosis: a preliminary assessment of their use and safety. Parassitologia. 2004;46(4):367-370. Available from: https://europepmc.org/article/med/16044692
- Giorgio A, Tarantino L, de SG, Francica G, Mariniello N, Farella N, et al. Hydatid liver cyst: an 11-year experience of treatment with percutaneous aspiration and ethanol injection. J Ultrasound Med. 2001;20(7):729-738. Available from: https://doi.org/10.7863/jum.2001.20.7.729
- Manterola C, Fernández O, Muñoz S, Vial M, Losada H, Carrasco R, et al. Laparoscopic pericystectomy for liver hydatid cysts. Surg Endosc. 2002;16(3):521-524. Available from: https://link.springer.com/article/10.1007/s00464-001-8125-7
- Goja S, Saha SK, Yadav SK, Tiwari A, Soin AS. Surgical approaches to hepatic hydatidosis ranging from partial cystectomy to liver transplantation. Ann Hepato-Biliary-Pancreat Surg. 2018;22:208–215. Available from: https://doi.org/10.14701/ahbps.2018.22.3.208
- Schipper HG, Lameris JS, van Delden OM, Rauws EA, Kager PA. Percutaneous evacuation (PEVAC) of multivesicular echinococcal cysts with or without cystobiliary fistulas which contain non-drainable material: first results of a modified PAIR method. Gut. 2002;50(5):718-723. Available from: https://gut.bmj.com/content/50/5/718.short
- Silva AM, Dias HV, Iria I, Fonseca MA, Valente M. Cystic echinococcosis in the liver: evaluation of percutaneous treatment. JGHR. 2015;21(4):1861-1868.
- Yagci G, Ustunsoz B, Kaymakcioglu N, Bozlar U, Gorgulu S, Simsek A, et al. Results of surgical, laparoscopic, and percutaneous treatment for cystic echinococcosis of the liver: 10 years experience with 355 patients. World J Surg. 2005;29(12):1670-1679. Available from: https://link.springer.com/article/10.1007/s00268-005-0058-1
- Sayek I, Onat D. Diagnosis and treatment of uncomplicated hydatid cyst of the liver. World J Surg. 2001;25:21-27. Available from: https://doi.org/10.1007/s002680020004
- Brunetti E, Tamarozzi F. Watch-and-wait approach for inactive echinococcal cysts: scoping review update since the issue of the WHO-IWGE Expert Consensus and current perspectives. Curr Opin Infect Dis. 2023;36(5):326-332. Available from: https://doi.org/10.1097/qco.0000000000000943
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
