International Journal of Clinical Endocrinology and Metabolism
Relationship of insulin resistance with microvascular and macrovascular complications, death rate and other factors in patients with type 2 diabetes: A case-control study
Gabrielė Čypaitė2, Diana Šimonienė2 and Emilė Rudminaitė1
2Department of Endocrinology, Lithuanian University of Health Sciences, Kaunas, Lithuania
Cite this as
Čypaitė G, Šimonienė D, Rudminaitė E. Relationship of insulin resistance with microvascular and macrovascular complications, death rate and other factors in patients with type 2 diabetes: A case-control study. Int J Clin Endocrinol Metab. 2024: 10(1): 006-013. Available from: 10.17352/ijcem.000060Copyright License
© 2024 Čypaitė G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Abstract: There is a lack of studies analyzing Severe Insulin Resistance (SIR) forms, especially from clinical aspects. The main objective of this research was to assess the relationship between micro- and macrovascular complications and the death rate of patients with SIR.
Methods: It was a partially prospective case-control study of 120 participants with T2DM. Patients with doses of insulin >1 IU/kg/day were considered to have SIR (case group), with an insulin requirement of <1 IU/kg/day - control group. Statistical analyses were performed.
Results:Regarding microvascular complications, an association was found between insulin doses and the rate of Diabetic Nephropathy (DN) in the case group compared with the control group (138 vs. 170 IU/day), p = 0.002. Men with T2DM and SIR were more likely to present with myocardial infarction than women (39.3% vs. 13.3%); p = 0.036. Subjects of the case group with lower eGDR (2.44 vs. 1.35) (or higher insulin resistance) were more likely to suffer a stroke, p = 0.003. Case group males were more likely than females to undergo coronary artery bypass graft surgery (21.4% vs. 3.3%); p = 0.048. Higher mortality was observed in the case group of patients with lower eGDR (1.01 vs. 1.85); p = 0.031.
Conclusion: As for microvascular outcomes, the rate of DP and DR was similar in both control and case groups. Only the rate of DN in the case group was associated with higher insulin doses. Macrovascular complications such as stroke, myocardial infarction, and bypass surgery were related to the male gender and SIR, as well as higher mortality according to eGDR.
Introduction
Nowadays, Diabetes Mellitus (DM) is considered to be an epidemic of the twenty-first century [1]. Insulin Resistance (IR) is a pivotal pathogenetic component of T2DM [2]. Whilst dysregulated insulin secretion and insulin resistance play a key role in type 2 DM pathogenesis, it is worth noting that other altered intra- and extrapancreatic mechanisms, such as inappropriate glucagon activity, also contribute to the final disease manifestation [2,3]. Still, the main focus of the treatment for type 2 Diabetes Mellitus (T2DM) is to reduce insulin resistance. However, despite insulin-sensitizing treatment, IR seems to be progressing further for most, with other factors contributing to the development of chronic complications of diabetes [4]. But for reasons that are not yet fully understood (no detailed analysis has been undertaken), some cases of IR progress to severe forms of IR. Treatment then becomes ineffective and has to be intensified with insulin therapy, which in turn can lead to further progression of SIR [5]. There is a lack of studies analyzing SIR forms, especially from clinical aspects. Without knowing the risk factors and health effects of SIR, the treatment of such patients who have T2DM with SIR remains ineffective, thus posing a challenge to the physician.
The prevalence of SIR is not known but is thought to account for 0.1% - 0.5% of all patients admitted to hospitals, due to poorly controlled diabetes [6]. Another study estimates the prevalence of SIR up to 7% [7]. SIR can be defined as a severely impaired response to the biological effects of insulin and is characterized by hyperinsulinemia and impaired glucose response to endogenous and exogenous insulin [8]. The higher the IR, the more pronounced both hyperglycemia and hyperinsulinemia are, both of which contribute to the development of chronic micro- and macrovascular DM complications [9].
There is evidence that people with diabetes have two to four times higher risks of Cardiovascular Diseases (CVDs) and death than those without diabetes [10]. It is estimated that diabetes is responsible for 11% of adult deaths each year [11]]. Higher mortality is associated with cardiovascular pathologies, which are the main cause of death in patients with DM, but IR is known to be one of the pathogenetic mechanisms of cardiovascular disease (CVD) [10-13]. It is known that IR is a suspected causative factor in a wide variety of diseases and is associated with all-cause or disease-specific mortality among nondiabetic persons [14] Moreover, rowing studies have shown that IR is associated with poor prognoses among patients with diabetes, although the relationship between IR and mortality remains poorly described in patients with T2DM, unlike those with T1DM [15]. One of the newest China studies has tried to evaluate the relationship between IR and all-cause mortality in the diabetic population. They used a novel metabolic score for insulin resistance (METS-IR) as an alternative marker of IR and demonstrated a non-linear association between METS-IR and all-cause, CVDs-related mortality in patients with diabetes [16]. Another study found that Insulin Sensitivity (IS) evaluated by eGDR predicts all-cause mortality in T2DM, independent of confounders [17].
Previously conducted studies have found that IR and DM not only reflect metabolic abnormalities but also predispositions to AH and vascular stiffness, and are linked to CVD [18]. A recent exploratory meta-analysis has shown that IR is an independent and strong risk factor for CVD [19-21]. It was found that the SIR-T2DM subtype was associated with the highest risk of developing Diabetic Kidney Disease (DKD) and Coronary Artery Disease (CAD) [22]. Moreover, 25% of people who have undergone CABG surgery for multivessel CAD have diabetes [23]. However, the real impact of SIR on the progress of diabetes and its complications is not known.
The most accurate method for determining IS is the Hyperinsulinemic-Euglycemic Clamp Test (HECT) [24]. This method is considered the gold standard for diagnosis but is impractical, invasive, and time-consuming. Moreover, it is not suitable for use in clinical practice. IR can be evaluated non-invasively by Insulin Sensitivity Indices (ISI) such as the Mcauley index (MCAi), which is a function of fasting insulin and triglycerides [25], but the test is not suitable for the diabetic population. The Homeostasis Model Assessment of IR (HOMA-IR) is widely used in most studies assessing IR [26,27]. However, this method is not reliable, especially if DM patients are treated with exogenous insulin. A more dependable clinical parameter to assess IS is to evaluate daily insulin requirements. Patients who require more than 1 IU/kg/day of exogenous insulin to maintain glycemic control are considered to be resistant to insulin [24].
Nevertheless, a growing number of studies have reported another indirect method of assessing IR: the estimated glucose disposal rate (eGDR). This method was first proposed to assess IR in type 1 diabetes mellitus (T1DM) patients, and later in T2DM [28]. This method is based on clinical variables, such as waist circumference (WC), HbA1c, and arterial hypertension (AH). It showed a good correlation with IR, compared to HECT [27,28]. Moreover, Helliwell, et al. found that low eGDR levels were related to both microvascular and macrovascular complications, independent of HbA1c [29].
The main objective of this research was to assess the relationship between microvascular, and macrovascular complications, and the death rate of patients with severe IR, and to evaluate other factors that may cause vascular complications in patients with T2DM.
Materials and methods
Patient selection
One hundred twenty patients, aged 18 or older, with T2DM, were included in the study. All patients included in this study were treated in the Endocrinology Department of the Lithuanian University of Health Sciences Kaunas Clinics. This study was conducted over three years (2018-2021).
Study design
The study was approved by the Kaunas Regional Biomedical Research Ethics Committee (BE-2-29, No SRI-01 version 2, 2017-05-17).
The study was conducted as a case-control study. Since death rates were calculated using a follow-up method, the study is considered partially prospective. In the absence of data on the prevalence of SIR, we have relied on a single source, which reported that, on average, 6% - 7% of patients with T2DM required high doses of insulin (defined as a total daily dose superior to 200 units) [X]. We chose this 7% frequency to calculate the sample size. The calculated study sample was 98 subjects with a 95% Confidence Interval (CI). Because the ratio of case-to-control subjects in our study was 1:1, a minimum of 50 subjects had to be included in each group. A total of 120 subjects were included in our study: 62 in the case group and 58 in the control group with T2DM.
Patients treated for at least three months with high doses of insulin (>1 IU/kg/day) who still did not reach a satisfying level of diabetes control (HbA1c ≥9%) were assigned to the SIR - case group: 58 patients were allotted to this group. Inversely, 62 patients who had been treated for at least 3 months with routine doses of insulin (<1 IU/kg/day) and who reached a satisfying level of diabetes control (HbA1c ≤8%) were assigned to the control group. In this case-control study, every patient who met the inclusion criteria was added to the case group and was matched by a patient in the control group, according to gender and Body Mass Index (BMI) (BMI differed ± 1 kg/m²). However, the age and duration of diabetes were not matched between both groups because of their strong correlation.
Inclusion and exclusion criteria
Inclusion criteria for this study were: patient aged 18 or older; T2DM diagnosed over a year ago; treatment with insulin in stable doses (long- and/or rapid-acting insulin), with or without metformin, for at least three months; consent given to participate in the study by signing the Informed Consent form.
The exclusion criteria were related to factors that change IS, such as oncological diseases (these include but are not limited to stage IV carcinomas and metastatic cancers); chronic or acute kidney failure (Glomerular Filtration Rate (GFR) <30 ml/min.); acute CAD (within the month preceding inclusion in the study); patients treated with glucocorticoids or hypoglycemic drugs (except metformin); patients who refused to continue participating in the study.
Collection and evaluation of data
At the time of enrolment, data were collected from subjects belonging to the case and control groups, using the following protocol:
- By interviewing patients using a standardized questionnaire about demographic data, diabetes, and confirmed diabetes complications, as well as CVD.
- By collecting information from medical documentation (age, sex, weight, height, BMI, duration of diabetes, information about current treatment, insulin doses, HbA1c, data about major cardiovascular events, and chronic complications of diabetes).
- Additional tests for diabetic complications were performed. The albumin-to-creatinine (a/c) ratio in a single morning urine sample was used to detect DN (normal range was assessed when a/c ratio was <3 mg/mmol); it was recommended not to eat at least eight hours before, not to inject basal insulin in the twelve hours leading up to, and not to inject rapid-acting insulin for at least four hours before the test. Blood samples were also tested for creatinine, lipid, insulin, glucose, and HbA1c levels.
- Patients were referred to an ophthalmologist for a consultation if they had not consulted in the last year.
- Insulin and glycemic tests were used to calculate the HOMA-IR index. The assessment of IR was performed in 2 ways:
- According to the mathematical formula of eGDR: 24.31 - (12.22 x WHR) - (3.29 x AH) - (0.57 x HbA1c), with WHR - waist/hip ratio; AH - arterial hypertension; HbA1c - glycated hemoglobin, expressed as a percentage. Calculated eGDR was expressed in milligrams per kilogram per minute (mg/kg/min). The lower the eGDR value, the higher the IR.
- According to the HOMA-IR index. A mathematical formula was also applied to calculate HOMA-IR: (fasting insulin concentration (mU/l) x fasting plasma glycemia (mmol/l)) / 22.5. IR was defined by HOMA-IR exceeding 2.5, according to literature recommendations.
Estimating the incidence of death: At the end of this case study, all the information on the participants of the study between 2018 and 2022 was reevaluated, and a high rate of mortality was noted. This partially prospective study aimed to compare the rate of death in the control and the case groups.
Mathematical statistical data analysis
Statistical analyses were performed with the data statistics package SPSS Statistics version 27. The normality of the sample distribution was confirmed by the Kolmogorov-Smirnov test, and the descriptive results of the subjects were presented as Mean (m) ± Standard Deviation (SD), with the categorical variables presented as frequency (%). In the absence of a normal distribution, the main data were presented as median and min-max. For quantitative data, the difference in means of normally distributed means was assessed by Student’s t-test for independent samples. The Chi-square (χ2) test was used to assess non-parametric criteria. Correlation analysis of parametric data was performed using Pearson’s correlation coefficient (r). The Mann-Whitney U test was used to compare two independent non-parametric sample means. To predict the dependent variables, binary logistic regression was performed. Variables included in the regression models were age, gender, HbA1c, BMI, diabetes duration, insulin level, and eGDR. Odds Ratios (OR) with 95% CI of significant factors were calculated. Receiver operating characteristic (ROC) curve analysis was chosen for the evaluation of the highest risk of MI in diabetic patients treated with insulin. The level of statistical significance was set as p < 0.05.
Results
Both groups had a similar distribution of men and women since the participants were matched by gender. Moreover, groups were also paired by BMI, resulting in a statistically insignificant difference in BMI between both groups. However, age differences were found: patients in the case group were significantly younger and had shorter diabetes duration (Table 1).
Because the inclusion criteria also considered insulin dosages and HbA1c levels, these differed between the groups. HOMA-IR and eGDR, which both measure IR, were also significantly different between groups, indicating that subjects with SIR had higher HOMA-IR and lower eGDR values (Table 1).
The distribution of microvascular complications of DM in the groups was assessed. A higher rate of DP in the case (SIR) group than in the control group was noticed (p = 0.055). There were no significant differences in rate concerning DR and DN (Table 2).
The detailed distribution of macrovascular complications of DM in the groups was also evaluated. Although the rate of macrovascular DM complications was higher in the case group, no significant difference was found between the groups (Table 2).
The higher rates of new death cases (overall mortality) were calculated for the group of people with severe IR and 2TDM, compared with a similar rate for people without severe IR and 2TDM. However, no significant difference was found, p = 0.273 (Table 2).
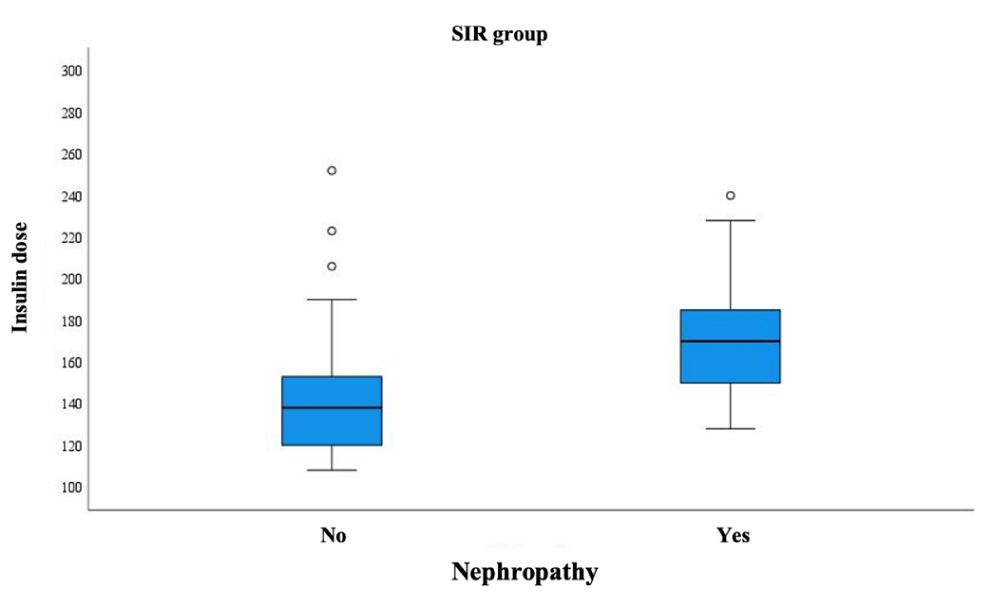
The association of microvascular DM complications with various factors (insulin dose, gender, age, DM duration, HbA1c, HOMA-IR, and eGDR) was assessed. DP was significantly more frequent in the control group with a longer duration of diabetes (14 years vs. 19 years); p = 0.022. An association was found between insulin dose and rate of DN in the SIR (case) group: DN was diagnosed more frequently in subjects who had been treated with high doses of insulin (138 vs. 170 IU/day); p = 0.002 (Figure 1). No such relationship was observed in the control group.
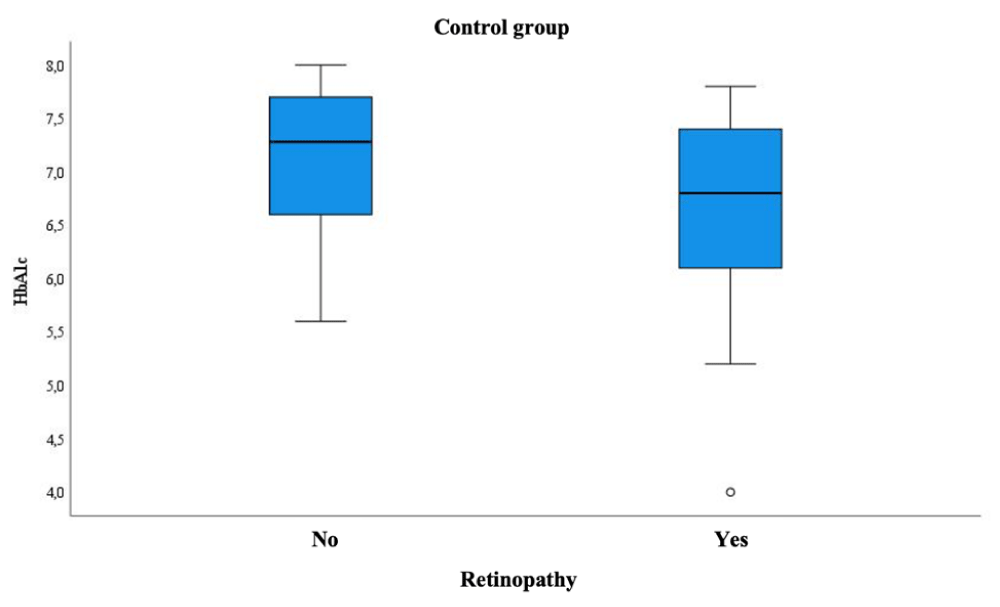
DR was more frequent in subjects of the control group who had better diabetic control, according to HbA1c (6.8% vs. 7.28%); p = 0.015 (Figure 2).
Using a logistic regression model, only DM duration had a significant impact on DR in the case group. By adopting a ROC curve, it was calculated that diabetes duration over 17 years in the case group (Area Under the Curve (AUC) 61.3%) increased DR risk by 2.504 [1.193-5.256] times, p = 0.014.
The association of macrovascular DM complications with various factors was also evaluated in different groups of subjects, using an adapted multivariate binary logistic regression model. In the case group, it was found that male gender and older age had an impact on MI (Table 3).
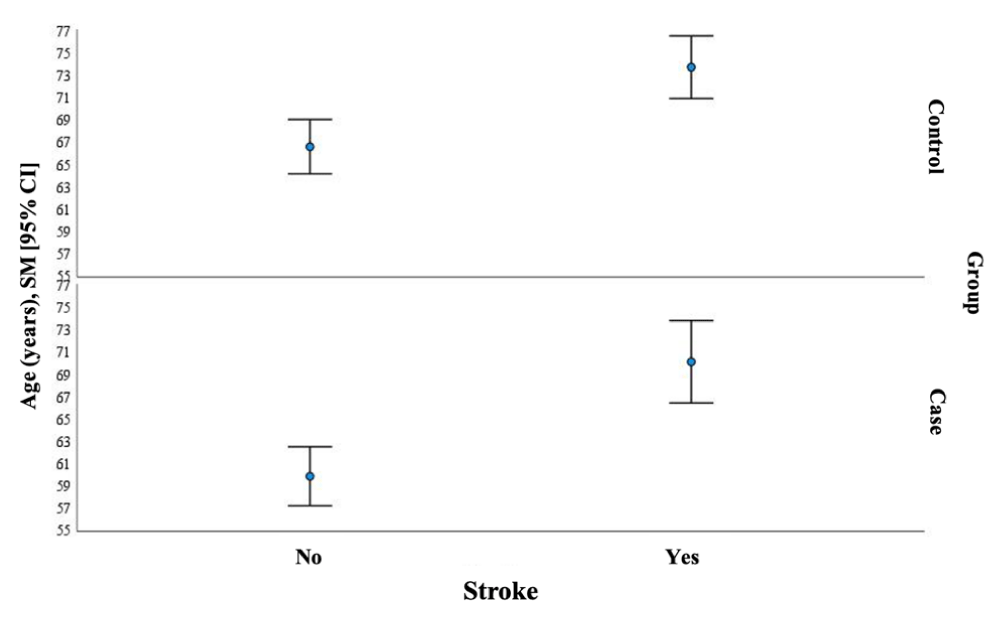
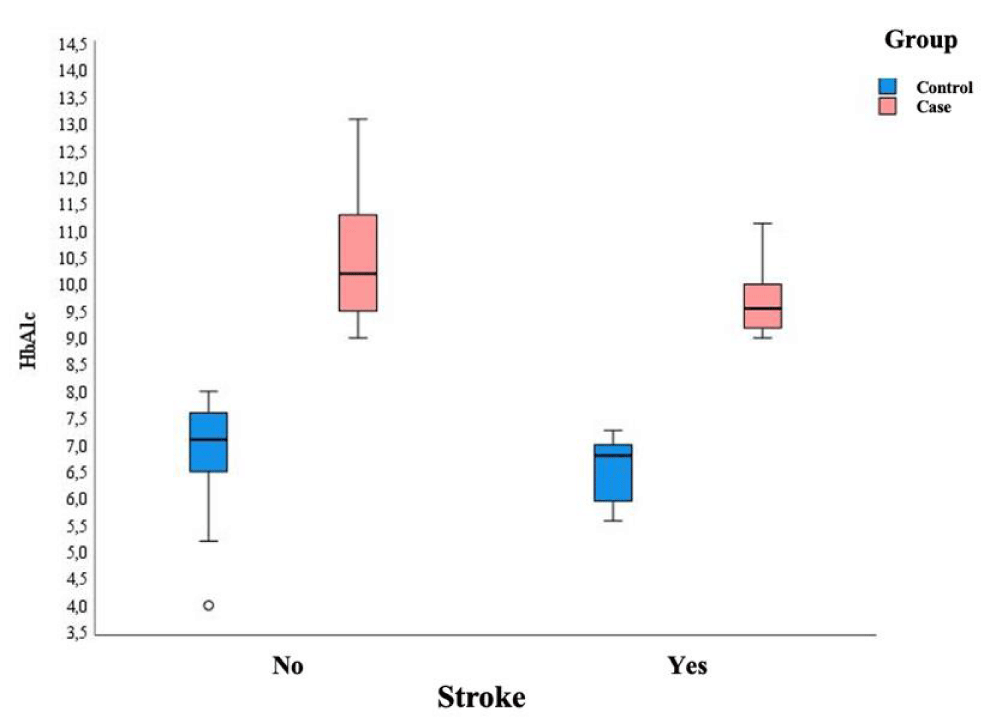
In both the case and control groups, older age and lower HbA1c were associated with a higher stroke rate: age 73.73 years vs. 66.59 years; p < 0.001 and HbA1c 6.80 % .vs. 7.10%; p = 0.038 in the control group (Figure 3). In the case group age 70.13 years vs. 59.84 years; p < 0.001 and HbA1c (9.55% vs. 10.20%; p = 0.05) (Figure 4).
There was an association between eGDR and stroke rate in the SIR group: subjects with lower eGDR (2.44 vs. 1.35) (or higher IR) were more likely to suffer a stroke, p = 0.003.
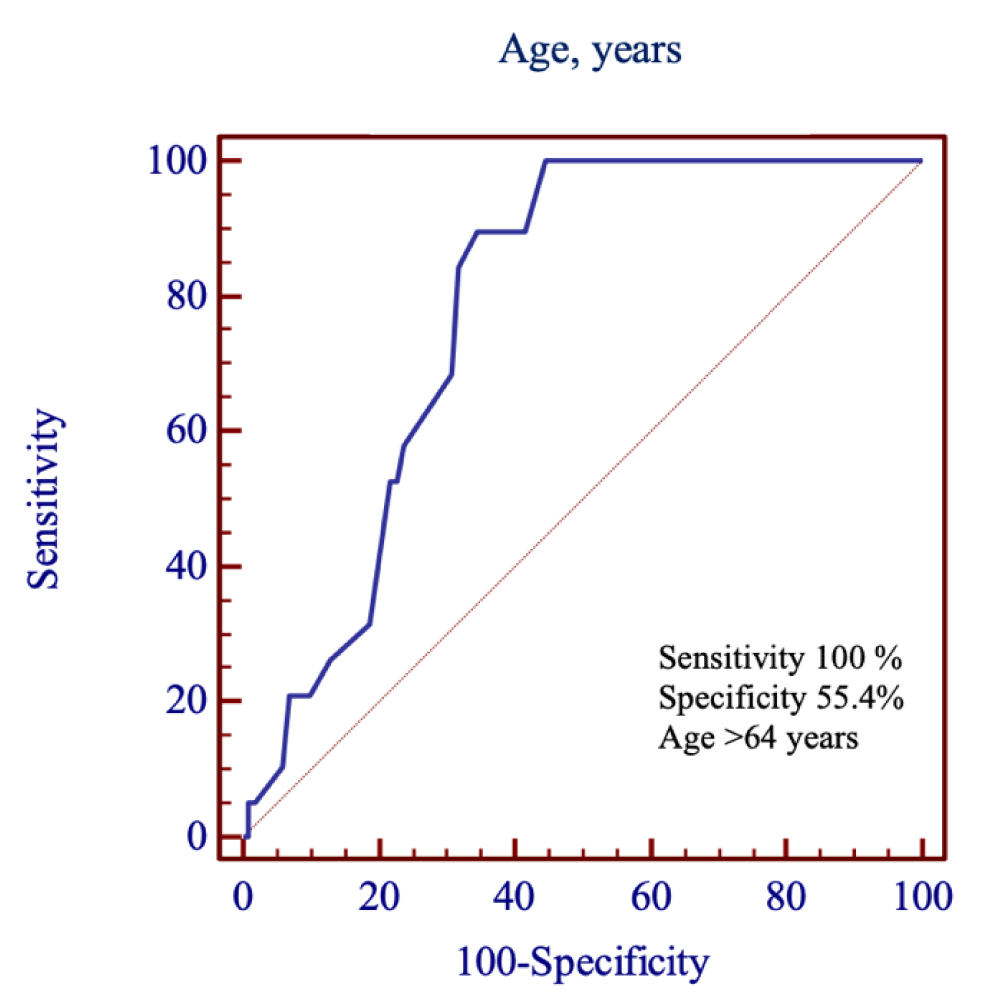
Using ROC curve analysis, we evaluated patients with high and low risk for strokes, based on the risk assessment model. In this sample, independent of IS, the area under the ROC curve (AUC=0.78, p < 0.001) showed that a diabetic patient aged over 64, (with 100% sensitivity and 55.4% specificity) had a 78.1% chance of having the highest risk rate of stroke (Figure 5).
After dividing the subjects into groups, a correlation was found between the rate of CABG surgery and gender in the case group. In the SIR group, males were more likely than females to undergo CABG (21.4% vs. 3.3%), p = 0.048. No such association was observed in the control group.
The relationship between various factors with the rate of death in both groups was assessed. In the case group, an association was found between higher mortality and eGDR. Higher mortality was observed in patients with T2DM and lower eGDR (1.01 vs. 1.85); p = 0.031 (Figure 6). In the control group, no association was observed between the death rate and eGDR.
Discussion
Our recent study is unique because of the different sights of diabetes and insulin resistance. There are still debates on the impact of high insulin dose demand on diabetes and cardiovascular diseases. Do these patients with Severe Insulin Resistance (SIR) are at increased risk for CVD, even death, do they need additional attention? The main goal of this study was to evaluate the relationship between diabetic complications and the death rate of patients with SIR and to evaluate other risk factors that cause cardiovascular complications in patients with T2DM. There are very few studies of this type, and these tend to focus more on treatment than on causal or outcomes analysis.
Our study did not show a significantly higher rate of chronic DM complications in the SIR group, although a trend was observed. Subjects with SIR had a higher daily insulin dose, as well as higher daily insulin requirement based on weight, HOMA-IR value, and lower eGDR, indicating that this group of subjects had all the features of IR.
Concerning IR and microvascular complications, a study published in 2022 by Xiayo He, et al. found that a group of subjects with T2DM and Metabolic Syndrome (MS), where IR is a key component of the complex syndrome, had a higher risk of microvascular complications than macrovascular complications, compared to T2DM patients without MS [30]. As previously stated, we did not find statistically significant results in our research to support the findings of the study described above. In our work, both microvascular and macrovascular complication rates were similar in the different groups, with no significant differences.
On the other hand, the latest study by Cuiqiao Meng, et al. which was published in 2023, has found that in Chinese individuals with T2DM, lower eGDR was independently associated with a higher risk of DR [27]. In our work, not only did we not find any correlation between DR and eGDR, but we also obtained results pointing to the contrary: DR was more frequent in subjects of the control group, who had better glycemic control according to HbA1c (6.8% vs. 7.28%), p = 0.015. Another extensive study, conducted by Emma Ahlqvist, et al. in 2018, showed similar results in other microvascular DM complications, like DKD: the increased incidence of DKD was despite reasonably low HbA1c [22]. These findings suggest that glucose-lowering therapy is not the only and also, not necessarily the best way to prevent microvascular complications.
The same study by Emma Ahlqvist, et al. that was mentioned above, where subjects were divided into diabetes subtypes, reported that severe forms of IR diabetes (SIRD) were associated with a high risk of DKD and hepatosteatosis [22]. However, this study defined SIR patients as subjects who had a high HOMA index, or required high insulin doses, but whose DM was adjusted (according to HbA1c). Therefore, there was a contradiction in the definition of SIR. In our study, the analyzed subtype was SIRD, where insulin was the only treatment and diabetes control was poor. Nevertheless, in our study an association between higher insulin dose and DKD was found as well: in the SIR group, DN was diagnosed more frequently in subjects who had been treated with high doses of insulin (138 vs. 170 IU/day); p = 0.002.
Our study found a very high rate of DP, whereas other literature shows significantly lower numbers. This may be related to the overdiagnosis of this diagnosis in Lithuania. The diagnosis is based on the patient’s subjective sensations and sensations evaluation tests (as vibration sensing estimation using a 128 Hz tuning fork, tactile sensory, and sensory evaluation of 10-g monofilament pressure), which are subjective and may be misleading if only one test was performed. Moreover, the subjects were enrolled in the study with a confirmed diagnosis of DP and we did not repeat the sensory evaluation tests, so it can be a reason for inaccurate results. On the other hand, the study was carried out in patients with a long duration of diabetes and comorbidities, and different insulin sensitivity, so the results may be as they are.
The link between IR and macrovascular complications remains an unfulfilled topic in people with T2DM, although current research is looking for relations between the rate of cardiovascular complications and various other factors in diabetes control. Large randomized control trials on diabetic patients, including the ACCORD, ADVANCE, and VADT trials have not shown any improvement in the risk of morbidity of macrovascular DM complications, despite better glucose control [31]. The same results were also reflected in our study: better glycemic control did not show any significantly lower rate of macrovascular complications in both the case and control groups. On the contrary, higher stroke rates were associated with lower HbA1c (better glucose control) in both groups. Lower HbA1c may be due to a higher rate of hypoglycemia.
The literature also provides more data on stroke and IR. In 2021, Alexander Zabala, et al. conducted a study including individuals with T2DM and showed that higher eGDR (decreased insulin resistance) was associated with a lower risk of stroke and death due to stroke [32]. Similar results were also found in our study: subjects with lower eGDR (2.44 vs. 1.35) (or higher IR) were more likely to suffer a stroke, p = 0.003. These conclusions confirm once again that IR is linked to the macrovascular complications of DM, and its identification could contribute to the development of more advanced medicine.
In addition, a recent retrospective cohort study, conducted by Matthew J. O’Brein, et al. where 132737 adults with T2DM were included, observed an association between insulin therapy and a higher prevalence of macrovascular complications, when used as second-line medications in adult T2DM patients [33]. In our study, no similar results were assessed. Therefore our results could contribute to the notion that the greatest effect in reducing cardiovascular morbidity may be directed towards reducing IR rather than hyperglycemia. To that point, multiple studies provide evidence that IR by itself is a major determinant of elevated cardiovascular risk in patients with T2DM [10].
In our study, we exclusively analyzed T2DM patients who received insulin therapy. We believe that medication and the reduction of IR, moreover consequently of cardiovascular complications, is an area where more research in the future is needed. The trend observed in the literature is that SGLT2 inhibitors, which reduce IR and improve glycemic control through glycosuria, also significantly impact and consequently reduce cardiovascular morbidity and mortality in T2DM patients [34].
Study strengths and limits
The advantage of our study is that similar studies about SIR and mortality rates have not yet been conducted. In this study, we looked for relations between IR and death, while also considering other factors in T2DM patients. As seen in our research, mortality rates in T2DM patients with severe IR were directly associated with lower eGDR scores: this suggests that higher IR increases the risk of death. However, further and larger studies are needed. When we performed the prospective study on the incidence of deaths, a sub-analysis of the causes of death was also included. Three main causes of death were identified: diabetes-related deaths, oncological, and others (included when no data were found on the cause of death, which could for example be CVD). Deaths due to CVD and other deaths accounted for the largest proportion, confirming the literature evidence that DM patients are most likely to die from CVD.
The main strength of our study is the uniqueness of the research in finding associations between SIR and complications. We were also looking for other risk factors in patients with T2DM with varying IS. The main limitation is the small sample size, but SIR is not a common pathology, and the power formula used to calculate the minimum sample size provided a sufficient sample for statistical calculations.
Conclusion
The rate of diabetic polyneuropathy and diabetic retinopathy was similar in both groups. The rate of diabetic nephropathy was associated with a higher insulin dose. The rate of macrovascular complications in patients with type 2 diabetes and severe insulin resistance was higher with male gender, older age, and uncompensated diabetes. Finally, higher mortality in patients with diabetes and severe IR was directly associated with lower eGDR scores, and indirectly with insulin resistance.
Take home message
Patients with type 2 diabetes and severe insulin resistance should be carefully observed by endocrinologists. Because these patients are prone to micro-and macrovascular complications and a higher rate of mortality.
We want to express our gratitude to all the participants who enthusiastically participated in our study.
Author contributions
Conceptualization, G.Č. and D.Š.; Methodology, D.Š.; Software, D.Š.; Validation, D.Š. and G.Č.; Formal analysis, D.Š.; Investigation, G.Č. and D.Š.; Resources, D.Š., and G.Č.; Data Curation, G.Č., D.Š., and E.R.; Writing - Original Draft Preparation, G.Č.; Writing - Review & Editing, G.Č., D.Š., and E.R.; Visualization, G.Č., D.Š., and E.R.; Supervision, D.Š.; Project Administration, G.Č.; Funding Acquisition, D.Š. All authors have read and agreed to the published version of the manuscript.
Informed consent statement
Informed consent was obtained from all patients before their involvement in the study. Written informed consent has been obtained from the patients to publish this paper.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or ethics committee) of the Kaunas Regional Committee on Ethics in Biomedical Research (BE-2-29, No SRI-01 version 2, 2017-05-17).
- Banday MZ, Sameer AS, Nissar S. Pathophysiology of diabetes: An overview. Avicenna J Med. 2020; 10(4):174-188. Available from: https://pubmed.ncbi.nlm.nih.gov/33437689/
- Cavaghan MK, Ehrmann DA, Polonsky KS. Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J Clin Invest. 2000; 106(3):329-333. Available from: https://pubmed.ncbi.nlm.nih.gov/10930434/
- Reed J, Bain S, Kanamarlapudi V. A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives. Diabetes Metab Syndr Obes. 2021; 14:3567-3602. Available from: https://pubmed.ncbi.nlm.nih.gov/34413662/
- Entezari M, Hashemi D, Taheriazam A, Zabolian A, Mohammadi S, Fakhri F, et al. AMPK signaling in diabetes mellitus, insulin resistance, and diabetic complications: A pre-clinical and clinical investigation. Biomed Pharmacother. 2022; 146:112563. Available from: https://doi.org/10.1016/j.biopha.2021.112563
- Juan CC, Fang VS, Kwok CF, Perng JC, Chou YC, Ho LT. Exogenous hyperinsulinemia causes insulin resistance, hyperendothelinemia, and subsequent hypertension in rats. Metabolism. 1999; 48(4):465-471. Available from: https://pubmed.ncbi.nlm.nih.gov/10206439/
- Trevisan M, Liu J, Bahsas FB, Menotti A. Syndrome X and mortality: a population-based study. Risk Factor and Life Expectancy Research Group. Am J Epidemiol. 1998; 148(10):958-966. Available from: https://pubmed.ncbi.nlm.nih.gov/9829867/
- Idris I, Peng X, He X, Liu D, Balogh E, Kaushik P, et al. The Trend of High-Dose Insulin Usage among Patients with Diabetes in the UK: A Retrospective Study. Diabetes Ther. 2018; 9(6):2245-2257. Available from: https://doi.org/10.1007/s13300-018-0515-0
- Angelidi AM, Filippaios A, Mantzoros CS. Severe insulin resistance syndromes. J Clin Invest. 2021; 131(4):1-13. Available from: https://pubmed.ncbi.nlm.nih.gov/33586681/
- Mendez CE, Walker RJ, Eiler CR, Mishriky BM, Egede LE. Insulin therapy in patients with type 2 diabetes and high insulin resistance is associated with increased risk of complications and mortality. Postgrad Med. 2019;000 131(6):376-382. Available from: https://pubmed.ncbi.nlm.nih.gov/31311382/
- Adeva-Andany MM, Martínez-Rodríguez J, González-Lucán M, Fernández-Fernández C, Castro-Quintela E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab Syndr Clin Res Rev. 2019; 13(2):1449-1455. Available from: https://pubmed.ncbi.nlm.nih.gov/31336505/
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018; 14(2):88-98. Available from: http://dx.doi.org/10.1038/nrendo.2017.151
- Lee SH, Park SY, Choi CS. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab J. 2022; 46(1):15-37. Available from: https://pubmed.ncbi.nlm.nih.gov/34965646/
- Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018; 17(1):1-14. Available from: https://cardiab.biomedcentral.com/articles/10.1186/s12933-018-0762-4
- Moshkovits Y, Rott D, Chetrit A, Dankner R. The association between insulin sensitivity indices, ECG findings and mortality: a 40-year cohort study. Cardiovasc Diabetol. 2021; 20(1):1-12. Available from: https://pubmed.ncbi.nlm.nih.gov/33957929/
- Garofolo M, Gualdani E, Scarale MG, Bianchi C, Aragona M, Campi F, et al. Insulin resistance and risk of major vascular events and all-cause mortality in type 1 diabetes: A 10-year follow-up study. Diabetes Care. 2020; 43(10):e139-41. Available from: https://pubmed.ncbi.nlm.nih.gov/32796028/
- Wang Z, Xie J, Wang J, Feng W, Liu N, Liu Y. Association Between a Novel Metabolic Score for Insulin Resistance and Mortality in People With Diabetes. Front Cardiovasc Med. 2022; 1-12. Available from: https://pubmed.ncbi.nlm.nih.gov/35647046/
- Penno G, Solini A, Orsi E, Bonora E, Fondelli C, Trevisan R, et al. Insulin resistance, diabetic kidney disease, and all-cause mortality in individuals with type 2 diabetes: a prospective cohort study. BMC Med. 2021; 19(1):1-13. Available from: https://pubmed.ncbi.nlm.nih.gov/33715620/
- Jia G, Sowers JR. Hypertension in Diabetes: An Update of Basic Mechanisms and Clinical Disease. Hypertension. 2021; 1197-1205. Available from: https://pubmed.ncbi.nlm.nih.gov/34601960/
- Chakraborty C, Doss CGP, Bandyopadhyay S, Agoramoorthy G. Influence of miRNA in insulin signaling pathway and insulin resistance: Micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip Rev RNA. 2014; 5(5):697-712. Available from: https://pubmed.ncbi.nlm.nih.gov/24944010/
- Ni Y, Fan D. Diabetes mellitus is a risk factor for low bone mass-related fractures. Medicine (Baltimore). 2017; 96(51):e8811. Available from: https://pubmed.ncbi.nlm.nih.gov/29390417/
- Zhang H, Hao Y, Manor B, Novak P, Milberg W, Zhang J, et al. Intranasal insulin enhanced resting-state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes. 2015; 64(3):1025-1034. Available from: https://pubmed.ncbi.nlm.nih.gov/25249577/
- Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018; 6(5):361-369. Available from: https://pubmed.ncbi.nlm.nih.gov/29503172/
- Nyström T, Holzmann MJ, Eliasson B, Svensson AM, Kuhl J, Sartipy U. Estimated glucose disposal rate and long-term survival in type 2 diabetes after coronary artery bypass grafting. Heart Vessels. 2017; 32(3):269-278. Available from: https://pubmed.ncbi.nlm.nih.gov/27401741/
- DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol. 1979; 6(3). Available from: https://pubmed.ncbi.nlm.nih.gov/382871/
- Ekblad LL, Rinne JO, Puukka P, Laine H, Ahtiluoto S, Sulkava R, et al. Insulin resistance predicts cognitive decline: An 11-year follow-up of a nationally representative adult population sample. Diabetes Care. 2017; 40(6):751-758. Available from: https://pubmed.ncbi.nlm.nih.gov/28381479/
- Ma L, Li Y. Cognitive function and insulin resistance in elderly patients with type 2 diabetes. Neurol Res. 2017; 39(3):259-263. Available from: http://dx.doi.org/10.1080/01616412.2017.1281199
- Meng C, Xing Y, Huo L, Ma H. Relationship between Estimated Glucose Disposal Rate and Type 2 Diabetic Retinopathy. Diabetes, Metab Syndr Obes. 2023; 16:807-818. Available from: https://pubmed.ncbi.nlm.nih.gov/36959899/
- Nyström T, Holzmann MJ, Eliasson B, Svensson AM, Sartipy U. Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes. Diabetes, Obes Metab. 2018; 20(3):556-563. Available from: https://pubmed.ncbi.nlm.nih.gov/28884949/
- Helliwell R, Warnes H, Kietsiriroje N, Campbell M, Birch R, Pearson SM, et al. Body mass index, estimated glucose disposal rate and vascular complications in type 1 diabetes: Beyond glycated hemoglobin. Diabet Med. 2021; 38(5):1-10. Available from: https://pubmed.ncbi.nlm.nih.gov/33502032/
- He S, Wang J, Zhang X, Qian X, Yan S, Wang W, et al. Long-term influence of type 2 diabetes and metabolic syndrome on all-cause and cardiovascular death, and microvascular and macrovascular complications in Chinese adults — A 30-year follow-up of the Da Qing diabetes study. Diabetes Res Clin Pract. 2022; 191:110048. Available from: https://doi.org/10.1016/j.diabres.2022.110048
- Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: A re-assessment of insulin therapy’s safety profile. Postgrad Med. 2016; 128(6):609-619. Available from: https://pubmed.ncbi.nlm.nih.gov/27210018/
- Zabala A, Darsalia V, Lind M, Svensson AM, Franzén S, Eliasson B, et al. Estimated glucose disposal rate and risk of stroke and mortality in type 2 diabetes: a nationwide cohort study. Cardiovasc Diabetol. 2021; 20(1):1-9. Available from: https://doi.org/10.1186/s12933-021-01394-4
- O’Brien MJ, Karam SL, Wallia A, Kang RH, Cooper AJ, Lancki N, et al. Association of Second-line Antidiabetic Medications with Cardiovascular Events among Insured Adults with Type 2 Diabetes. JAMA Netw Open. 2018; 1(8):1-15. https://pubmed.ncbi.nlm.nih.gov/30646315/
- Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, et al. Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus. Circulation. 2019; 139(22):2528-2536. Available from: https://pubmed.ncbi.nlm.nih.gov/30882238/
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
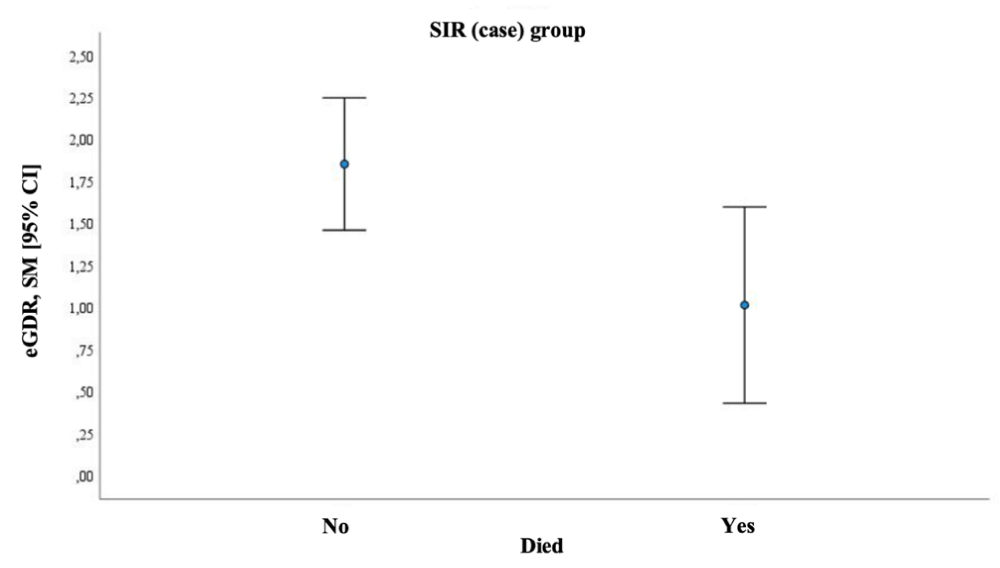


 Save to Mendeley
Save to Mendeley
