Archives of Clinical Gastroenterology
Epidermoid Cyst arising within an Intrapancreatic Accessory Spleen [ECIPAS] mimicking a pancreatic mucinous cystic neoplasm-a case report with literature review
Pramath Kakodkar1,2, Dana Diudea1,2 and Rani Kanthan1,2*
2Saskatchewan Health Authority, Saskatoon, Canada
Cite this as
Kakodkar P, Diudea D, Kanthan R (2024) Epidermoid Cyst arising within an Intrapancreatic Accessory Spleen [ECIPAS] mimicking a pancreatic mucinous cystic neoplasm-a case report with literature review. Arch Clin Gastroenterol 10(2): 010-018. DOI: 10.17352/2455-2283.000122Copyright License
© 2024 Kakodkar P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and r eproduction in any medium, provided the original author and source are credited.Background: Accessory spleens are congenital embryological aberrations usually found within the splenic hilum with no clinical significance. An Intra Pancreatic Accessory Spleen (IPAS) is an uncommon benign pancreatic lesion encountered clinically. The occurrence of an Epidermoid Cyst arising within an IPAS (ECIPAS) is exceedingly rare and is often misdiagnosed as a pancreatic pathology such as mucinous cystic neoplasm, cystic degeneration within a solid tumor such as a neuroendocrine tumor, or a lymph node.
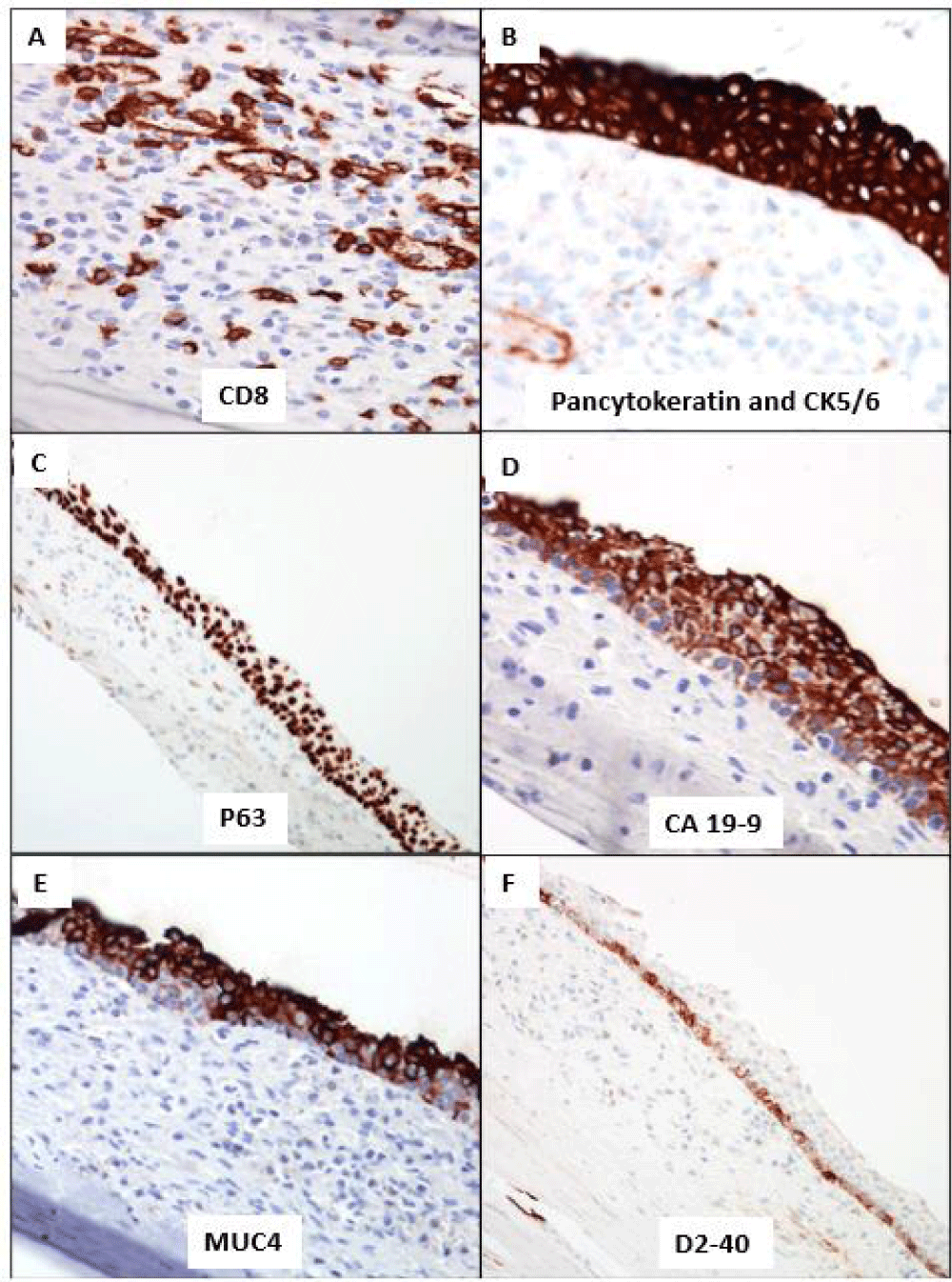
Case report: A 68-year-old male presented with intermittent post-prandial abdominal pain for over 2 years. Abdominal computer tomography identified a 5.2 cm calcified cyst within the pancreatic tail and a mucinous pancreatic neoplasm/pancreatic pseudocyst was considered in the differential diagnosis. The results of endoscopic ultrasound and fine needle aspiration were indeterminate. Due to persistent abdominal pain, the patient underwent a laparoscopic distal pancreatectomy with splenectomy. A gross examination of the distal pancreatic/splenectomy specimen confirmed a well-demarcated cystic lesion with brownish fluid within the pancreatic tail. Microscopic examination revealed a nonpathological pancreas separated by a fibrous capsule with a large cyst arising within an intrapancreatic accessory spleen. The cyst was lined with multilayered non-keratinized stratified squamous epithelium positive for pancytokeratin, CA 19-9, CK5/6, and p63 with no lymphocytic infiltrates and absent hair/ dermal appendages confirming an epidermoid cyst. CD8 outlined the dendritic network of the littoral cells of the splenic tissue in the cyst wall. Post-operative follow-up at 6 weeks was uneventful.
Conclusion: The preoperative diagnosis of ECIPAS is extremely difficult as this entity shares overlapping radiological features with other cystic lesions such as mucinous pancreatic cysts. It is important for anatomic pathologists to recognize and consider the intrapancreatic compartment as a possible site for accessory spleen. As histopathology is the final determinant of this diagnosis, increased clinical awareness with an accurate diagnosis of this entity may prevent patients from unnecessary surveillance and/or extensive oncological-based surgical resection.
Introduction
Accessory spleens are incidentally found at autopsy in 12.1% of cases [1]. Approximately, 9.3 - 22% of these accessory spleens were identified within the pancreatic tail [1-3]. Epidermoid cyst within an intrapancreatic accessory spleen (ECIPAS) is a rare cystic neoplasm first described by Davidson, et al. in 1980 [4]. ECIPAS predominantly behaves as a benign lesion and to date, there is only one documented case of ECIPAS transformation to squamous cell carcinoma [5].
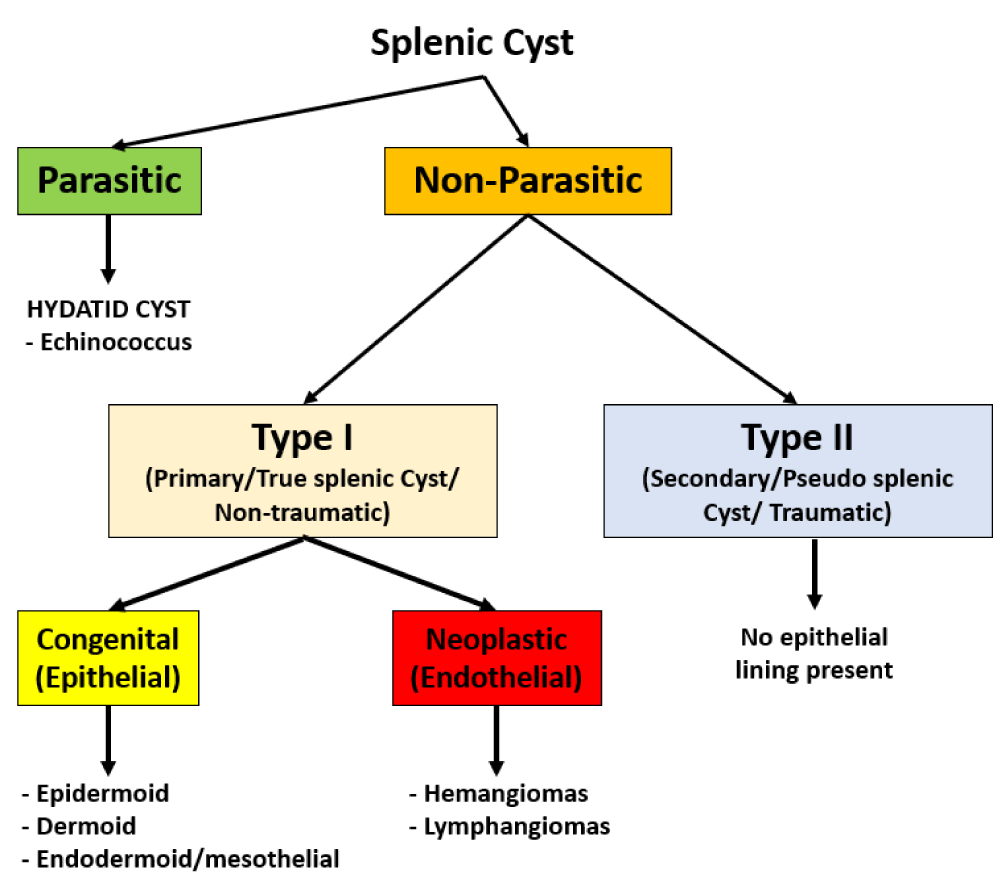
Radiological diagnosis of this entity is extremely challenging as it is difficult to differentiate ECIPAS from other ‘pancreatic cystic neoplasms’ such as Mucinous Cystic Neoplasm (MCN), cystic degeneration within a Neuroendocrine Tumour (NET) and Solid Pseudopapillary Tumour (SPT) [6,7]. Due to the malignant neoplastic potential of these entities, most of the patients with ECIPAS undergo surgical resection. Contrastingly, if a cystic lesion is identified within an accessory spleen preoperatively then the differential diagnosis is similar to that of splenic cysts as outlined in Figure 1.
In this study, we report the case of a 68-year-old with ECIPAS who underwent distal pancreatectomy and splenectomy due to suspicion of MCN. A comprehensive literature review is also performed to summarize the clinical and pathological insights into cases with ECIPAS. The ancillary objective of our case report is to highlight this entity for a surgical pathologist to consider within their differential diagnosis of cystic lesions within the pancreas and to encourage clinical awareness for exploration of surgical alternatives such as cyst excision with splenic preservation in lieu of distal pancreatectomy with total splenectomy [7].
Case presentation/report
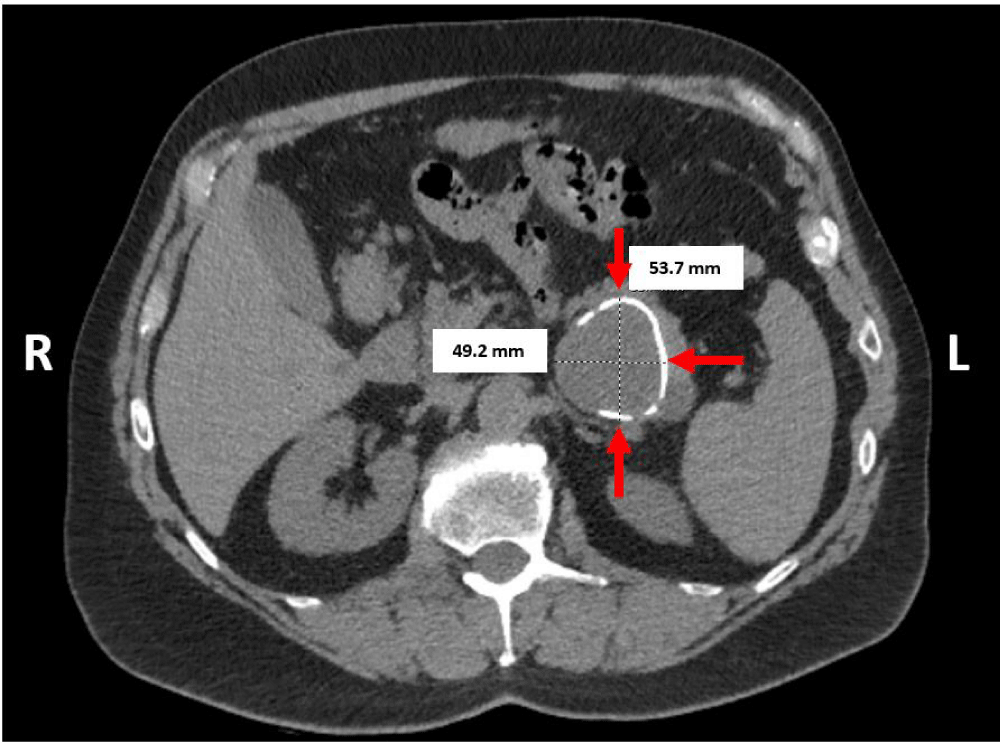
A 68-year-old otherwise healthy male presented with dull intermittent post-prandial epigastric pain radiating to the left flank for over 2 years. His physical examination was unremarkable. Biochemical tests were within normal limits except for elevated ferritin. Serum carbohydrate antigen 19-9 (CA 19-9) and Carcinoembryonic Antigen (CEA) tests were not performed. His routine laboratory data was within normal limits. Abdominal Computed Tomography (CT) showed a 5.4 x 4.9 cm cystic mass with partial calcification within the pancreatic tail (Figure 2). The radiology-based differential diagnosis included mucinous pancreatic neoplasm or pancreatic pseudocyst.
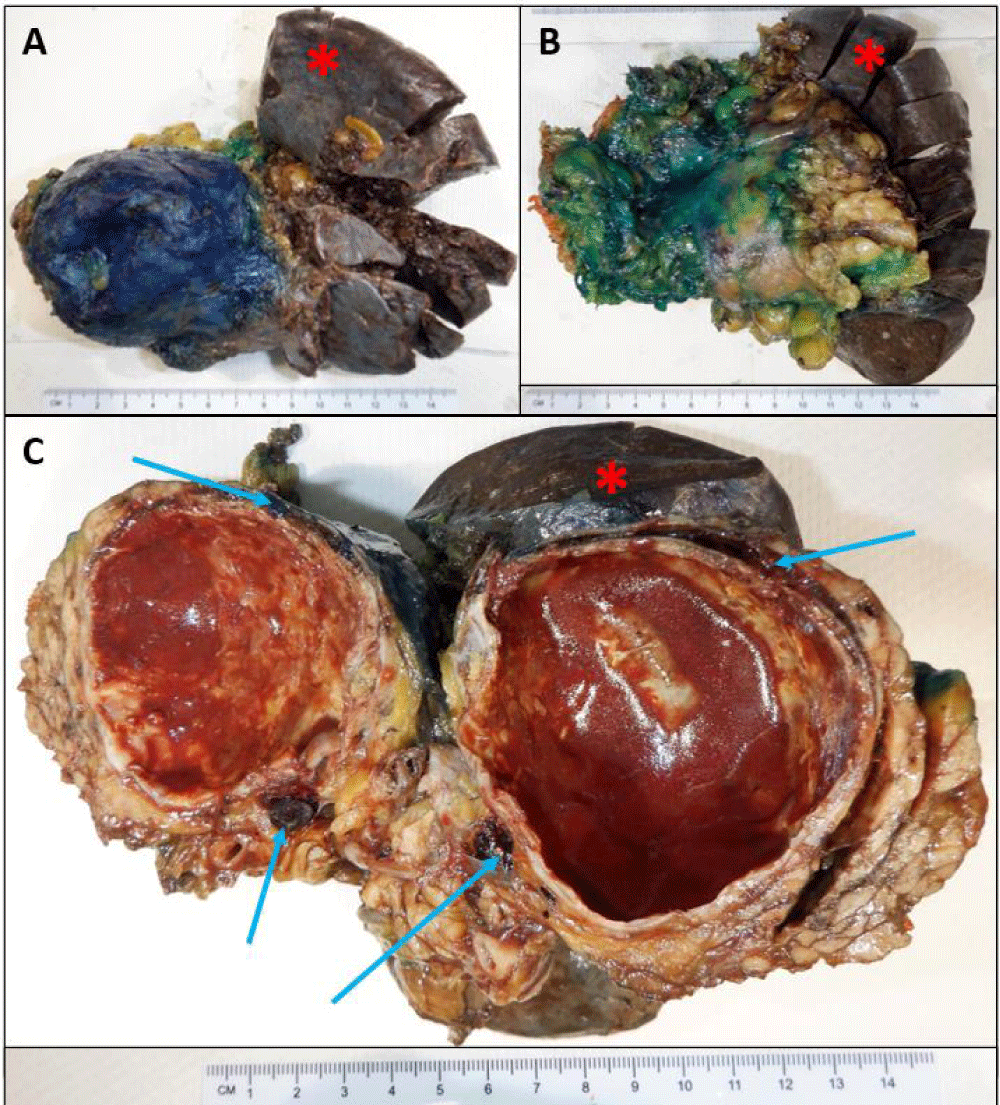
Fine needle aspiration via endoscopic ultrasound was attempted with indeterminate results. The patient underwent a laparoscopic distal pancreatectomy and splenectomy for query mucinous neoplasm of the pancreas. On gross examination, a cystic, partially calcified well-demarcated mass measuring 5.7 x 5.3 x 5.2 cm containing red-brown fluid was identified within the tail of the pancreas separate from the native spleen as seen in Figures 3A-C. Additional three firm well-defined nodules measuring 0.3 cm, 0.9 cm and 0.4 cm were identified abutting the splenic capsule and around the cyst as highlighted by arrows in Figure 3C.
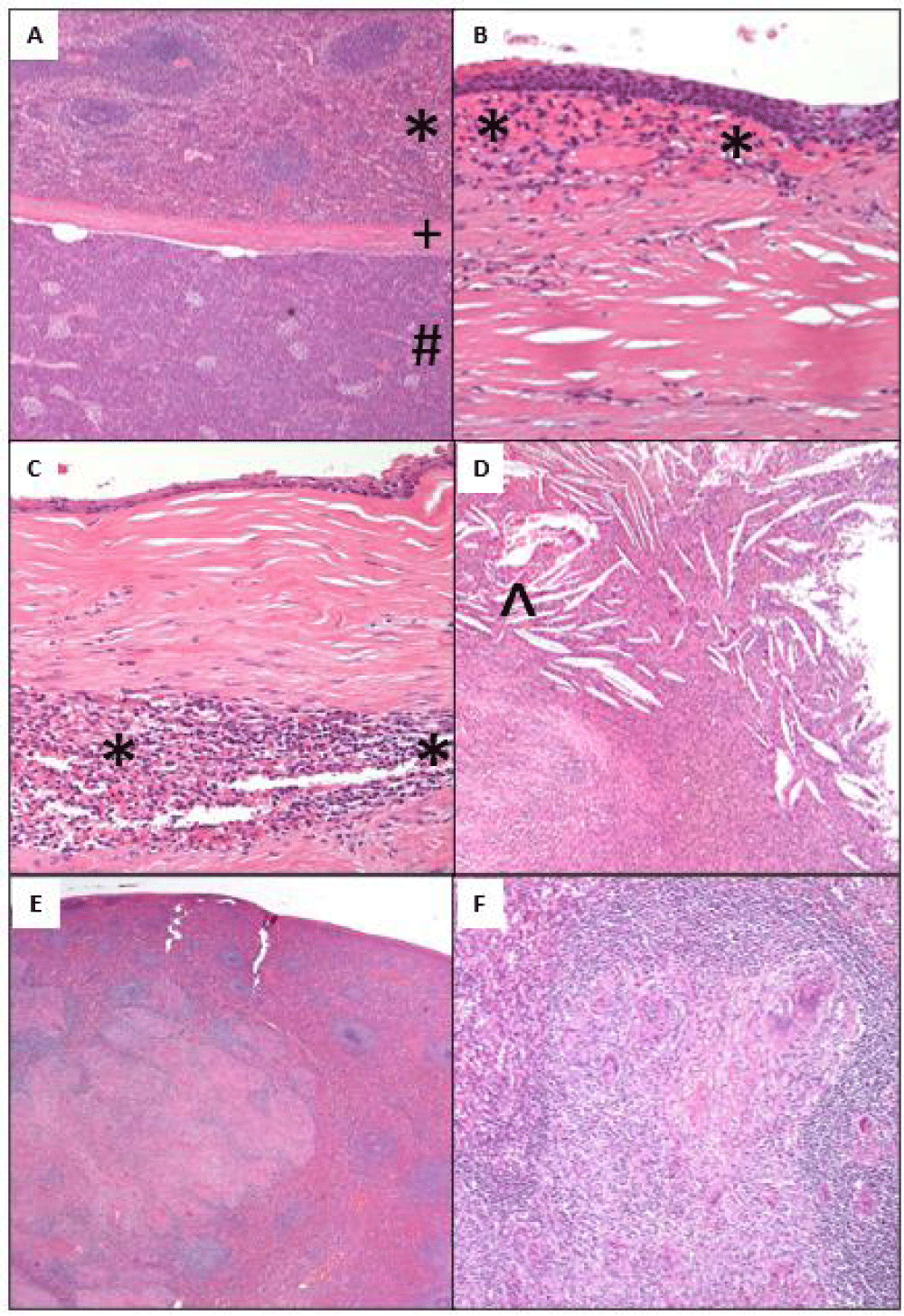
Microscopic examination revealed an unremarkable pancreas with the presence of splenic tissue [*] inside the pancreas [#] separated by a thick fibrous capsule [+] confirming the presence of an intrapancreatic accessory spleen containing a cyst with a thick fibrous wall with foci of dystrophic calcifications (Figure 4A). The cyst was lined by non-keratinized Stratified Squamous Epithelium (SSE) (Figure 4B). In other areas, the cyst lining was denuded to a cuboidal monolayer with subepithelial fibrosis, and underlying accessory splenic tissue [*] was identified within the cyst wall (Figure 4B,C). No ovarian stroma was identified. The cyst wall showed no evidence of lymphocytic infiltrates or dermal appendages. There was evidence of cyst rupture with chronic inflammation and cholesterol clefts [^] (Figure 4D). No hair was present within the cyst. The nodules identified on gross were three accessory spleens. The native spleen had large coalescing granulomas (Figure 4E) containing multinucleated giant cells (Figure 4F). Acid-fast bacilli (AFB) stain, periodic acid-Schiff (PAS), and Grocott’s methenamine silver (GMS) special stains showed no evidence of acid-fast bacilli or fungal microorganisms.
The Immunohistochemistry (IHC) stains for CD8 confirmed the classic dendritic pattern of staining of the littoral cells within the accessory splenic tissue (Figure 5A). The cyst SSE lining showed block positivity for pancytokeratin and CK5/6 (Figure 5B). P63 was also expressed with a preferential gradient pattern of stronger basal to suprabasal (Figure 5C). The superficial layers of the SSE lining showed positivity for CA 19-9 and MUC4 (Figure 5D and 5E). D2-40 expression was limited to the basal layer of cells with no expression in superficial layers (Figure 5F). There was no expression of Calretinin and WT1. The overall histology and IHC immunophenotype confirmed the presence of an Epidermoid Cyst arising within an Intrapancreatic Accessory Spleen [ECIPAS]. The patient remained asymptomatic, and his post-operative follow-up was unremarkable.
Discussion
ECIPAS is a rare entity and has been reported in 59 patients within the existing English literature (Table 1). It is also notable these 49 publications analyzed in our literature review were from Japan (25), United States (9), South Korea (5), China (4), United Kingdom (1), Australia (1), Greece (1), Netherlands (1), and Singapore (2). To the best of our knowledge, this is the first case report from Canada.
ECIPAS was commonly identified in the 47 ± 13 years-old population [Range: 12-70] and shows a female gender predilection (F:M = 1.4: 1). These patients were either asymptomatic with incidental cystic lesions on their radiological imaging (55.9%, n = 33 out of 59) or presented with abdominal pain (38.9%, n = 23 out of 59). In cases with localized abdominal pain (n = 15), left-sided (42.8%, n = 6 out of 14) or epigastric (35.7%, n = 5 out of 14) pain was common. Abdominal ultrasound and computer tomography (CT) were first-line radiological studies ordered in these patients. Notable findings included cystic lesions within the pancreas (86.4%, n = 51 out of 59) with rim enhancement, calcifications, and no anatomic continuity with the spleen (27.2%, n = 12 out of 44). The majority of the ECIPAS were located within the pancreatic tail (98.3%, n = 58 out of 59). As radiological findings lack specificity, the preoperative diagnosis is varied as seen in Table 1 with pancreatic malignant cystic neoplasms (74.6%, n = 44 out of 59) and mucinous cystic neoplasm (42.8%, n = 18 out of 42) being the predominant preoperative diagnoses.
The majority of these ECIPAS patients underwent extensive surgical resection in the form of distal pancreatectomy with splenectomy (54.2%, n = 32 out of 59) or distal pancreatectomy (38.9%, n = 23 out of 59). Two patients had ECIPAS treated by robot-assisted spleen-preserving distal pancreatectomy while another ECIPAS within the head of the pancreas underwent a pylorus-preserving pancreaticoduodenectomy (PPPD) [7,44,46]. Our patient underwent oncological-based surgery of distal pancreatectomy with splenectomy since MCN was the preoperative diagnosis. A possible suggestion to circumvent proceeding to these radical invasive surgical procedures is to obtain an intraoperative frozen section: biopsy the cyst wall to obtain a tissue diagnosis of ECIPAS to guide further management that may result in non-oncological resections with splenic preservation. However, parameters such as cyst size, location, and clinical presentation with symptoms will need to be considered together with an acknowledgement of resection acting as a preventative intervention against future cyst rupture, hemorrhage, infection, or recurrence for developing management plans.
In the last 5 years, there has been an increasing utilization of endoscopic ultrasound (EUS) procedures multiplexed with contrast enhancement or sulfur hexafluoride microbubbles to assist with the delineation of the margin of this pancreatic tail cystic lesion from the spleen. This preoperative assessment for clear margin delineation resulted in spleen-preserving distal pancreatomy in nine patients [44,48,50,51]. Interestingly, one patient underwent non-operative management based on the radiological findings in EUS and superparamagnetic iron oxide imaging (SPIO) which highlighted the subjacent parenchyma of the cyst showed signal hyperintensity similar to the spleen [52]. Utilizing these novel contrast-enhanced EUS and adequate fine needle sampling of the cystic lining that confirmed benign squamous cells, and CD8 positive splenic microvessel clusters with numerous lymphocytes in two cases did yield a preoperative diagnosis of ECIPAS [47,51,52].
The ECIPAS gross specimens resected in the literature review were distributed between multilocular (46.5%, n = 27 out of 59) and unilocular (47.4%, n = 28 out of 59) cysts with white to brown serous fluid contents, and a median largest cyst diameter of 3.0 cm [Range: 1-15 cm]. The histological findings in these cases showed a characteristic triad of non-neoplastic pancreatic tissue with hyalinized fibrous capsule and accessory splenic tissue identified by histology and the dendritic staining pattern of CD8 highlighting the littoral cells of the spleen. The cysts were lined with stratified squamous or cuboidal monolayer cells, and these cyst-lining cells were positive for CA 19-9 and cytokeratins (CK7, CK19, CK5/6, AE1/AE3). The majority of the cysts were lined by non-keratinizing squamous cells apart from 4 cases with keratinizing squamous cells [13,14,30,37]. However, it is to be noted that the cyst lining can be denuded and therefore the above triad cannot be mandated for accurate diagnosis of ECIPAS [53]. Many cases also report histological evidence of cyst rupture such as hematoma, cholesterol clefts, and giant cells [4,11,36,42,50]. As the cyst wall lining and contents are key determinants for cyst classification, the absence of hair and skin appendages in the cystic lesion and no lymphocytic infiltrates are the key histopathological features that differentiate an epidermoid cyst from a dermoid cyst and lymphoepithelial cyst respectively.
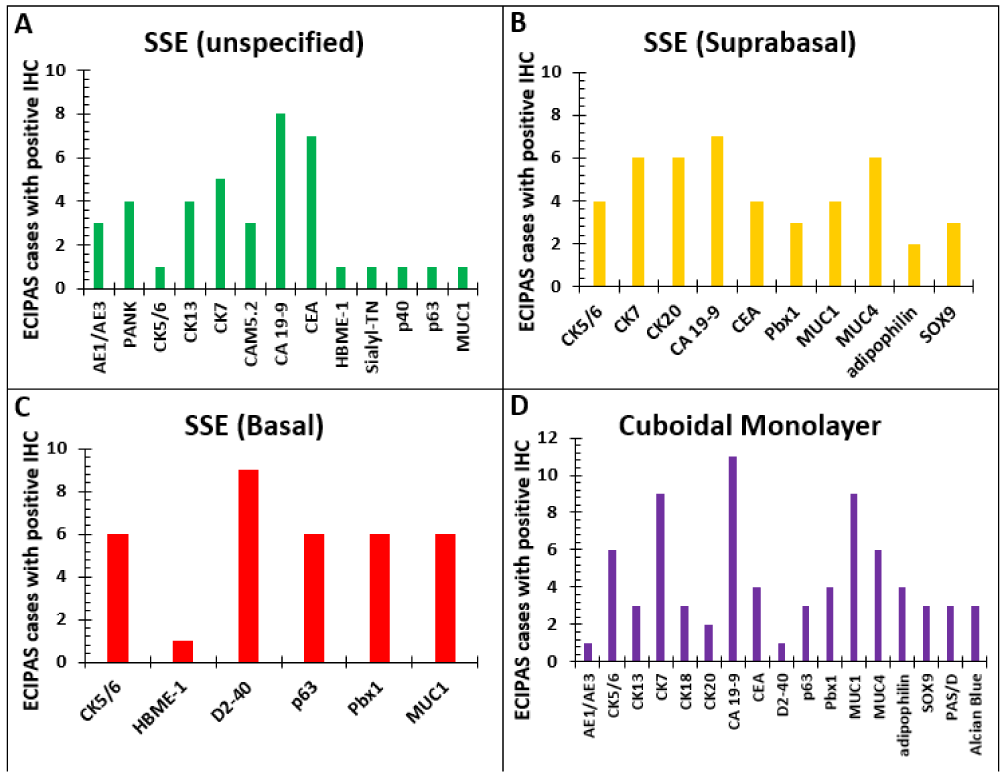
The Immunohistochemistry (IHC) profile for ECIPAS was reported in 33 cases in the literature. A summary of the pattern of staining in the ECIPAS lining for each reported IHC for patients in our literature review is shown in Figure 6. Overall, stratified squamous epithelium (SSE) of the ECIPAS stained positive predominantly with CA 19-9 (n = 8), CEA (n = 7), and CK7 (n = 6). The suprabasal SSE was notably positive for CA 19-9 (n = 7), CK7 (n = 6), CK 20 (n = 6) and MUC4 (n = 6). Contrastingly, basal SSE was commonly positive for D2-40 (n = 9), CK5/6 (n = 6), p63 (n = 6), Pbx1 (n = 6) and MUC1 (n = 6). Lastly, the cuboidal monolayer was predominantly positive for CA 19-9 (n = 11), CK7 (n = 9), CK5/6 (n = 6), MUC1 (n = 6), and MUC4 (n = 6). ECIPAS cases were predominantly negative for Calretinin (n = 7), WT-1 (n = 6), and vimentin (n = 4). We utilized Pancytokeratin, CK5/6, CK19, CK7, CA 19-9, MUC4, p63, Calretinin, WT1, and D2-40 for our IHC workup of ECIPAS and the pattern of staining was concordant with the reported literature. It is interesting to note that though Calretinin and WT1 are not expressed in this lesion there is basal expression of D2-40. Although D2-40 is a selective marker for germ cells and the lymphatic endothelium it is also a novel marker for cells with a mesothelial phenotype [54]. This is in keeping with the postulated congenital, primary, non-traumatic origin of these cysts that are favored to occur as a result of mesothelial cell misfolding in utero with invagination of the capsular surface mesothelium with subsequent cystic expansion and squamous metaplastic changes over time [55,56]. The rupture of the splenic capsule and small cystic spaces covered by mesothelium is probably one of the first steps in the genesis of the epithelial cyst of the spleen. This incorporation of mesothelial cells is a prominent determining factor in identifying splenic epidermoid cysts and is seen in their positive immunohistochemistry pattern of staining [56]. Our case report shows that the basal pattern of D2-40 indicates a primitive mesothelial cell phenotype. Contrastingly, the lack of Calretinin or WT1 expression indicates the absence of a more differentiated mesothelial phenotype.
It has been theorized that CA 19-9 and CEA are produced by the ECIPAS lining and disseminate into systemic circulation via trauma or elevated intracystic pressure. The changes in the serum CA 19-9 were recorded in our literature review (n = 36) highlighting the possible utility of this test in the preoperative work-up of ECIPAS. Elevated (61.1%, n = 22 out of 36) or normal (38.9%, n = 14 out of 36) levels of serum CA 19-9 were detected in these ECIPAS patients. There was no correlation between the gross size of ECIPAS and the elevation of CA 19-9. Interestingly, 10 of these patients with elevated serum CA 19-9 had a normalization of their serum CA 19-9 post-resection [6,13,14,17,18,20,22,33,39,40]. This post-operative decrease in CA 19-9 can be used to evaluate the extent of resection. Contrastingly, elevated levels of serum CEA were only concordant with CA 19-9 in 4 cases [13,14,30,37]. Therefore, intraoperative cyst fluid assessment for elevation in CA 19-9 paired with frozen section diagnosis can be considered as additional options to promote management options of spleen preserving surgical resections. All patients in the literature review were stable during their postoperative follow-up period. There is only one documented case of ECIPAS transformation to squamous cell carcinoma after 6 years from initial diagnosis [5]. Therefore, ECIPAS is predominantly a benign entity, and long-term follow-up with abdominal ultrasound paired with CA 19-9 may be utilized in monitoring these patients when clinically warranted.
The histogenesis of ECIPAS is yet to be discerned but the literature proposes two theories. The first theory suggests that similar to the histogenesis of splenic epidermoid cysts (SEC) there is an invagination of capsular mesothelial cells which undergo metaplastic change and cyst remodeling [55,56]. A genetic defect of mesothelial migration is considered the cause. While most are sporadic, rarely there is a familial occurrence [57]. The second theory suggests that these epithelial cysts are teratomatous derivatives or inclusion of fetal SSE [58]. Therefore, an exploration into the molecular mechanism of SEC may provide further insights into the histogenesis of ECIPAS. A genome-wide linkage and exome analyses study identified variants within the calcium-binding Epidermal Growth Factor (cb-EGF) like the domain of the fibulin 6 (HMCN1) protein in cases of SEC [59]. Fibulin 6 is an extracellular protein that is involved in cell adhesion, migration, and proliferation and is highly expressed in splenic tissue [60,61]. It is possible that instability in the HMCN1 structure can result in the misplacement of epithelial cells during developmental stages leading to SEC formation [57]. These variants in HMCN1 are also associated with age-related macular degeneration [62]. No biological studies exploring the function of human HMCN1 are available in the literature. Studies in the Zebrafish show that Hmcn1 mutation results in fin blistering due to cysts lined with epithelial cells [63]. HMCN1 could be a potential molecular candidate for future studies to explore the underlying molecular mechanism underlying the pathogenesis of ECIPAS.
Conclusion
The preoperative diagnosis of ECIPAS is extremely difficult as this entity shares overlapping radiological features with malignant pancreatic neoplasms and other cystic lesions such as mucinous pancreatic cysts thus resulting in oncological-based surgical resections. It is important for anatomic pathologists to recognize and consider the intrapancreatic compartment as a possible site for accessory spleen. This together with the evaluation of the cyst lining at the frozen section may facilitate consideration of cystectomy in relevant cases. The extent of surgical resection can be evaluated by measuring serum CA 19-9 in select cases. As histopathology is the final determinant of this diagnosis, increased clinical awareness with an accurate diagnosis of this entity may prevent patients from unnecessary surveillance and/or extensive oncological-based surgical resections.
- Halpert B, Gyorkey F. Lesions observed in accessory spleens of 311 patients. Am J Clin Pathol. 1959 Aug;32(2):165-8. doi: 10.1093/ajcp/32.2.165. PMID: 13670140.
- Mortelé KJ, Mortelé B, Silverman SG. CT features of the accessory spleen. AJR Am J Roentgenol. 2004 Dec;183(6):1653-7. doi: 10.2214/ajr.183.6.01831653. Erratum in: AJR Am J Roentgenol. 2005 Jan;184(1):348. PMID: 15547205.
- Unver Dogan N, Uysal II, Demirci S, Dogan KH, Kolcu G. Accessory spleens at autopsy. Clin Anat. 2011 Sep;24(6):757-62. doi: 10.1002/ca.21146. Epub 2011 Mar 3. PMID: 21374729.
- Davidson ED, Campbell WG, Hersh T. Epidermoid splenic cyst occurring in an intrapancreatic accessory spleen. Dig Dis Sci. 1980 Dec;25(12):964-7. doi: 10.1007/BF01308048. PMID: 7449592.
- Wang J, Kang WJ, Cho H. Malignant Transformation of an Epidermoid Cyst in an Intrapancreatic Accessory Spleen: A Case Report. Nucl Med Mol Imaging. 2020 Feb;54(1):58-60. doi: 10.1007/s13139-019-00631-9. Epub 2019 Dec 21. PMID: 32206133; PMCID: PMC7062970.
- Yamanishi H, Kumagi T, Yokota T, Koizumi M, Azemoto N, Watanabe J, Mizuno Y, Sugita A, Abe M, Ikeda Y, Matsuura B, Hiasa Y, Onji M. Epithelial cyst arising in an intrapancreatic accessory spleen: a diagnostic dilemma. Intern Med. 2011;50(18):1947-52. doi: 10.2169/internalmedicine.50.5340. Epub 2011 Sep 15. PMID: 21921374.
- van Dijck WP, Groot VP, Brosens LA, Hagendoorn J, Rinkes IH, van Leeuwen MS, Molenaar IQ. Rare Case of an Epithelial Cyst in an Intrapancreatic Accessory Spleen Treated by Robot-Assisted Spleen Preserving Distal Pancreatectomy. Case Rep Gastrointest Med. 2016;2016:9475897. doi: 10.1155/2016/9475897. Epub 2016 Oct 25. PMID: 27847657; PMCID: PMC5099494.
- Hanada M, Kimura M, Kitada M, Nakajima T, Yamada K, Yoshii M. Epidermoid cyst of accessory spleen. Acta Pathol Jpn. 1981 Sep;31(5):863-72. doi: 10.1111/j.1440-1827.1981.tb02811.x. PMID: 7304174.
- Morohoshi T, Hamamoto T, Kunimura T, Yoshida E, Kanda M, Funo K, Nagayama T, Maeda M, Araki S. Epidermoid cyst derived from an accessory spleen in the pancreas. A case report with literature survey. Acta Pathol Jpn. 1991 Dec;41(12):916-21. doi: 10.1111/j.1440-1827.1991.tb01639.x. PMID: 1785350.
- Nakae Y, Hayakawa T, Kondo T, Shibata T, Kitagawa M, Sakai Y, Sobajima H, Ishiguro H, Tanikawa M, Nimura Y, et al. Epidermoid cyst occurring in a pancreatic accessory spleen. J Clin Gastroenterol. 1991 Jun;13(3):362-4. doi: 10.1097/00004836-199106000-00024. PMID: 2066557.
- Tang X, Tanaka Y, Tsutsumi Y. Epithelial inclusion cysts in an intrapancreatic accessory spleen. Pathol Int. 1994 Aug;44(8):652-4. doi: 10.1111/j.1440-1827.1994.tb01726.x. PMID: 7952152.
- Furukawa H, Kosuge T, Kanai Y, Mukai K. Epidermoid cyst in an intrapancreatic accessory spleen: CT and pathologic findings. AJR Am J Roentgenol. 1998 Jul;171(1):271. doi: 10.2214/ajr.171.1.9648813. PMID: 9648813.
- Higaki K, Jimi A, Watanabe J, Kusaba A, Kojiro M. Epidermoid cyst of the spleen with CA19-9 or carcinoembryonic antigen productions: report of three cases. Am J Surg Pathol. 1998 Jun;22(6):704-8. doi: 10.1097/00000478-199806000-00007. PMID: 9630177.
- Tateyama H, Tada T, Murase T, Fujitake S, Eimoto T. Lymphoepithelial cyst and epidermoid cyst of the accessory spleen in the pancreas. Mod Pathol. 1998 Dec;11(12):1171-7. PMID: 9872647.
- Sasou S, Nakamura S, Inomata M. Epithelial splenic cysts in an intrapancreatic accessory spleen and spleen. Pathol Int. 1999 Dec;49(12):1078-83. doi: 10.1046/j.1440-1827.1999.00983.x. PMID: 10632928.
- Tsutsumi S, Kojima T, Fukai Y, Kanoh K, Shimura T, Mochiki E, Kato R, Asao T, Kuwano H. Epidermoid cyst of an intrapancreatic accessory spleen--a case report. Hepatogastroenterology. 2000 Sep-Oct;47(35):1462-4. PMID: 11100377.
- Horibe Y, Murakami M, Yamao K, Imaeda Y, Tashiro K, Kasahara M. Epithelial inclusion cyst (epidermoid cyst) formation with epithelioid cell granuloma in an intrapancreatic accessory spleen. Pathol Int. 2001 Jan;51(1):50-4. doi: 10.1046/j.1440-1827.2001.01155.x. PMID: 11148465.
- Sonomura T, Kataoka S, Chikugo T, Hirooka T, Makimoto S, Nakamoto T, Sato M. Epidermoid cyst originating from an intrapancreatic accessory spleen. Abdom Imaging. 2002 Sep-Oct;27(5):560-2. doi: 10.1007/s00261-001-0145-1. PMID: 12172998.
- Fink AM, Kulkarni S, Crowley P, Crameri JA. Epidermoid cyst in a pancreatic accessory spleen mimicking an infected abdominal cyst in a child. AJR Am J Roentgenol. 2002 Jul;179(1):206-8. doi: 10.2214/ajr.179.1.1790206. PMID: 12076937.
- Yokomizo H, Hifumi M, Yamane T, Hirata T, Terakura H, Murata K, Fujita H, Matsukane H. Epidermoid cyst of an accessory spleen at the pancreatic tail: diagnostic value of MRI. Abdom Imaging. 2002 Sep-Oct;27(5):557-9. doi: 10.1007/s00261-001-0055-2. PMID: 12172997.
- Kanazawa H, Kamiya J, Nagino M, Uesaka K, Yuasa N, Oda K, Arai T, Nishio H, Nimura Y. Epidermoid cyst in an intrapancreatic accessory spleen: a case report. J Hepatobiliary Pancreat Surg. 2004;11(1):61-3. doi: 10.1007/s00534-003-0844-9. PMID: 15754048.
- Watanabe H, Yamaguchi Y, Ohtsubo K, Mouri H, Motoo Y, Yamashita K, Minamoto T, Gabata T, Sawabu N. Epidermoid cyst of the intrapancreatic accessory spleen producing CA19‐9. Digestive Endoscopy. 2004 Jul;16(3):244-8. Doi: 10.1111/j.1443-1661.2004.00347.x
- Won JK, Lee YJ, Kang GH. Epithelial cysts in the intrapancreatic accessory spleen that clinically mimic pancreatic cystic tumor. Korean J Pathol. 2005 Nov;39(39):437-41.
- Ru K, Kalra A, Ucci A. Epidermoid cyst of intrapancreatic accessory spleen. Dig Dis Sci. 2007 May;52(5):1229-32. doi: 10.1007/s10620-006-9376-x. PMID: 17385039.
- Itano O, Shiraga N, Kouta E, Iri H, Tanaka K, Hattori H, Suzuki F, Otaka H. Epidermoid cyst originating from an intrapancreatic accessory spleen. J Hepatobiliary Pancreat Surg. 2008;15(4):436-9. doi: 10.1007/s00534-007-1243-4. Epub 2008 Aug 1. PMID: 18670847.
- Servais EL, Sarkaria IS, Solomon GJ, Gumpeni P, Lieberman MD. Giant epidermoid cyst within an intrapancreatic accessory spleen mimicking a cystic neoplasm of the pancreas: case report and review of the literature. Pancreas. 2008 Jan;36(1):98-100. doi: 10.1097/MPA.0b013e3181359e36. PMID: 18192891.
- Gleeson FC, Kendrick ML, Chari ST, Zhang L, Levy MJ. Epidermoid accessory splenic cyst masquerading as a pancreatic mucinous cystic neoplasm. Endoscopy. 2008 Sep;40 Suppl 2:E141-2. doi: 10.1055/s-2007-995735. PMID: 18633876.
- Zhang Z, Wang JC. An epithelial splenic cyst in an intrapancreatic accessory spleen. A case report. JOP. 2009 Nov;10(6):664-6. PMID: 19890189.
- Reiss G, Sickel JZ, See-Tho K, Ramrakhiani S. Intrapancreatic splenic cyst mimicking pancreatic cystic neoplasm diagnosed by EUS-FNA. Gastrointest Endosc. 2009 Sep;70(3):557-8; discussion 558. doi: 10.1016/j.gie.2009.04.050. Epub 2009 Jul 15. PMID: 19608182.
- Choi SK, Ahn SI, Hong KC, Kim SJ, Kim TS, Woo ZH, Shin SH. A case of epidermoid cyst of the intrapancreatic accessory spleen. J Korean Med Sci. 2000 Oct;15(5):589-92. doi: 10.3346/jkms.2000.15.5.589. PMID: 11068999; PMCID: PMC3054692.
- Kato S, Mori H, Zakimi M, Yamada K, Chinen K, Arashiro M, Shinoura S, Kikuchi K, Murakami T, Kunishima F. Epidermoid Cyst in an Intrapancreatic Accessory Spleen: Case Report and Literature Review of the Preoperative Imaging Findings. Intern Med. 2016 Dec;55(23):3445-3452. doi: 10.2169/internalmedicine.55.7140. PMID: 27904107; PMCID: PMC5216141.
- Itano O, Chiba N, Wada T, Yuasa Y, Sato T, Ishikawa H, Koyama Y, Matsui H, Kitagawa Y. Laparoscopic resection of an epidermoid cyst originating from an intrapancreatic accessory spleen: report of a case. Surg Today. 2010;40(1):72-5. doi: 10.1007/s00595-009-4006-9. Epub 2009 Dec 29. PMID: 20037845.
- Iwasaki Y, Tagaya N, Nakagawa A, Kita J, Imura J, Fujimori T, Kubota K. Laparoscopic resection of epidermoid cyst arising from an intrapancreatic accessory spleen: a case report with a review of the literature. Surg Laparosc Endosc Percutan Tech. 2011 Oct;21(5):e275-9. doi: 10.1097/SLE.0b013e31822dd14a. PMID: 22002295.
- Urakami A, Yoshida K, Hirabayashi Y, Kubota H, Yamashita K, Hirai T, Tsunoda T. Laparoscopy-assisted spleen-preserving pancreatic resection for epidermoid cyst in an intrapancreatic accessory spleen. Asian J Endosc Surg. 2011 Nov;4(4):185-8. doi: 10.1111/j.1758-5910.2011.00102.x. PMID: 22776306.
- Khashab MA, Canto MI, Singh VK, Hruban RH, Makary MA, Giday S. Endosonographic and elastographic features of a rare epidermoid cyst of an intrapancreatic accessory spleen. Endoscopy. 2011 May;43 Suppl 2 UCTN:E193-4. doi: 10.1055/s-0030-1256272. PMID: 21590599.
- Horn AJ, Lele SM. Epidermoid cyst occurring within an intrapancreatic accessory spleen. A case report and review of the literature. JOP. 2011 May 6;12(3):279-82. PMID: 21546709.
- Harris AC, Chaudry MA, Menzies D, Conn PC. Laparoscopic resection of an epidermoid cyst within an intrapancreatic accessory spleen: a case report and review article. Surg Laparosc Endosc Percutan Tech. 2012 Aug;22(4):e246-9. doi: 10.1097/SLE.0b013e31825b3761. PMID: 22874714.
- Hong R, Choi N, Sun K, Lim S, Han Y. Epidermoid cyst arising from an intrapancreatic accessory spleen: A case report and review of the literature. Oncol Lett. 2013 Feb;5(2):469-472. doi: 10.3892/ol.2012.1061. Epub 2012 Dec 4. PMID: 23420784; PMCID: PMC3573018.
- Lee CL, Di Y, Jiang YJ, Jin C, Fu DL. Epidermoid cyst of intrapancreatic accessory spleen: A case report and literature review. World J Surg Proced 2013; 3(3): 54-59. doi: 10.5412/wjsp.v3.i3.54.
- Zavras N, Machairas N, Foukas P, Lazaris A, Patapis P, Machairas A. Epidermoid cyst of an intrapancreatic accessory spleen: a case report and literature review. World J Surg Oncol. 2014 Apr 10;12:92. doi: 10.1186/1477-7819-12-92. PMID: 24721745; PMCID: PMC3986436.
- Fujii M, Yoshioka M, Shiode J. Two Cases of an Epidermoid Cyst Developing in an Intrapancreatic Accessory Spleen Identified during Laparoscopic Distal Pancreatectomy. Intern Med. 2016;55(21):3137-3141. doi: 10.2169/internalmedicine.55.7141. Epub 2016 Nov 1. PMID: 27803407; PMCID: PMC5140862.
- Kato S, Mori H, Zakimi M, Yamada K, Chinen K, Arashiro M, Shinoura S, Kikuchi K, Murakami T, Kunishima F. Epidermoid Cyst in an Intrapancreatic Accessory Spleen: Case Report and Literature Review of the Preoperative Imaging Findings. Intern Med. 2016;55(23):3445-3452. doi: 10.2169/internalmedicine.55.7140. Epub 2016 Dec 1. PMID: 27904107; PMCID: PMC5216141.
- Paredes A, Beal EW, Dillhoff ME. Epidermoid cyst within an intrapancreatic accessory spleen. BMJ Case Rep. 2018 Apr 5;2018:bcr2017223600. doi: 10.1136/bcr-2017-223600. PMID: 29622708; PMCID: PMC5893975.
- Tan HJ, Neo WL, Lee SY, Goh BKP, Kam JH. Epidermal Inclusion Cyst in an Intra-pancreatic Accessory Spleen: a Differential Diagnosis for Pancreatic Cystic Neoplasms and Review of the Literature. J Gastrointest Cancer. 2019 Jun;50(2):308-314. doi: 10.1007/s12029-017-0002-2. PMID: 28889365.
- Kato T, Matsuo Y, Ueda G, Aoyama Y, Omi K, Hayashi Y, Imafuji H, Saito K, Tsuboi K, Morimoto M, Ogawa R, Takahashi H, Kato H, Yoshida M, Naitoh I, Hayashi K, Takahashi S, Takiguchi S. Epithelial cyst arising in an intrapancreatic accessory spleen: a case report of robotic surgery and review of minimally invasive treatment. BMC Surg. 2020 Oct 31;20(1):263. doi: 10.1186/s12893-020-00927-0. PMID: 33129283; PMCID: PMC7603683.
- Ko HJ, Shim JR, Lee TB, Choi BH, Lee JH, Ryu JH, Yang K. Epidermoid cyst in an intrapancreatic accessory spleen in the pancreas head: a case report. BMC Gastroenterol. 2020 Nov 20;20(1):392. doi: 10.1186/s12876-020-01540-4. PMID: 33218300; PMCID: PMC7678289.
- Poola S, Laks S, Kragel P, Regan K. Intrapancreatic Accessory Spleen Masquerading as a Pancreatic Mucinous Neoplasm. Surg J (N Y). 2020 Jun 16;6(2):e128-e130. doi: 10.1055/s-0040-1710342. PMID: 32566751; PMCID: PMC7297644.
- Zheng X, Zhou B, Sun JQ, Jin M, Yan S. Laparoscopic spleen-preserving distal pancreatectomy for epidermoid cyst in an intrapancreatic accessory spleen: A case report. Medicine (Baltimore). 2021 Jul 2;100(26):e26379. doi: 10.1097/MD.0000000000026379. PMID: 34190154; PMCID: PMC8257839.
- Wee JJ, Vu CKF, Ding CSL, Shelat VG. Intrapancreatic accessory spleen with an epidermoid cyst: a malignant mimicry. BMJ Case Rep. 2022 Feb 14;15(2):e247737. doi: 10.1136/bcr-2021-247737. PMID: 35165128; PMCID: PMC8845195.
- Sumida S, Ichimura-Shimizu M, Miyakami Y, Kakimoto T, Kobayashi T, Saijo Y, Matsumoto M, Ogawa H, Oya T, Bando Y, Uehara H, Taira S, Shimada M, Tsuneyama K. Histological and immunohistochemical analysis of epithelial cells in epidermoid cysts in intrapancreatic accessory spleen. J Med Invest. 2023;70(1.2):251-259. doi: 10.2152/jmi.70.251. PMID: 37164730.
- Sun Y, Luo J, Huang F, Huang P, Yu R. Giant Cyst of Intra-Pancreatic Accessory Spleen Mimicking a Malignant Cystic Neoplasm of the Pancreas: a case report. Pancreas. 2024 Jun 24. doi: 10.1097/MPA.0000000000002383. Epub ahead of print. PMID: 38904669.
- Minami R, Nakahodo J, Tabata H, Chiba K, Horiguchi S, Kamisawa T. Epidermoid cyst in an intrapancreatic accessory spleen diagnosed by contrast-enhanced EUS and EUS-FNA (with video). Endoscopic Ultrasound. 2024 Feb; 13(1):49-51. doi:10.1097/eus.0000000000000047
- Shan GD, Chen WG, Hu FL, Chen LH, Yu JH, Zhu HT, Gao QQ, Xu GQ. A spontaneous hematoma arising within an intrapancreatic accessory spleen: A case report and literature review. Medicine (Baltimore). 2017 Oct;96(41):e8092. doi: 10.1097/MD.0000000000008092. PMID: 29019877; PMCID: PMC5662300.
- Chu AY, Litzky LA, Pasha TL, Acs G, Zhang PJ. Utility of D2-40, a novel mesothelial marker, in the diagnosis of malignant mesothelioma. Mod Pathol. 2005 Jan;18(1):105-10. doi: 10.1038/modpathol.3800259. PMID: 15389250.
- Ough YD, Nash HR, Wood DA. Mesothelial cysts of the spleen with squamous metaplasia. Am J Clin Pathol. 1981 Nov;76(5):666-9. doi: 10.1093/ajcp/76.5.666. PMID: 7293981.
- Bürrig KF. Epithelial (true) splenic cysts. Pathogenesis of the mesothelial and so-called epidermoid cyst of the spleen. Am J Surg Pathol. 1988 Apr;12(4):275-81. PMID: 3354753.
- Kubo M, Yamane M, Miyatani K, Udaka T, Mizuta M, Shirakawa K. Familial epidermoid cysts of the spleen: report of two cases. Surg Today. 2006;36(9):853-6. doi: 10.1007/s00595-006-3244-3. PMID: 16937296.
- Lifschitz-Mercer B, Open M, Kushnir I, Czernobilsky B. Epidermoid cyst of the spleen: a cytokeratin profile with comparison to other squamous epithelia. Virchows Arch. 1994;424(2):213-6. doi: 10.1007/BF00193502. PMID: 7514079.
- Omer WH, Narita A, Hosomichi K, Mitsunaga S, Hayashi Y, Yamashita A, Krasniqi A, Iwasaki Y, Kimura M, Inoue I. Genome-wide linkage and exome analyses identify variants of HMCN1 for splenic epidermoid cyst. BMC Med Genet. 2014 Oct 23;15:115. doi: 10.1186/s12881-014-0115-4. PMID: 25338956; PMCID: PMC4258954.
- de Vega S, Iwamoto T, Yamada Y. Fibulins: multiple roles in matrix structures and tissue functions. Cell Mol Life Sci. 2009 Jun;66(11-12):1890-902. doi: 10.1007/s00018-009-8632-6. PMID: 19189051; PMCID: PMC11115505.
- Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, Wernerus H, Björling L, Ponten F. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010 Dec;28(12):1248-50. doi: 10.1038/nbt1210-1248. PMID: 21139605.
- Weeks DE, Conley YP, Tsai HJ, Mah TS, Schmidt S, Postel EA, Agarwal A, Haines JL, Pericak-Vance MA, Rosenfeld PJ, Paul TO, Eller AW, Morse LS, Dailey JP, Ferrell RE, Gorin MB. Age-related maculopathy: a genomewide scan with continued evidence of susceptibility loci within the 1q31, 10q26, and 17q25 regions. Am J Hum Genet. 2004 Aug;75(2):174-89. doi: 10.1086/422476. Epub 2004 May 27. PMID: 15168325; PMCID: PMC1216053.
- Carney TJ, Feitosa NM, Sonntag C, Slanchev K, Kluger J, Kiyozumi D, Gebauer JM, Coffin Talbot J, Kimmel CB, Sekiguchi K, Wagener R, Schwarz H, Ingham PW, Hammerschmidt M. Genetic analysis of fin development in zebrafish identifies furin and hemicentin1 as potential novel fraser syndrome disease genes. PLoS Genet. 2010 Apr 15;6(4):e1000907. doi: 10.1371/journal.pgen.1000907. PMID: 20419147; PMCID: PMC2855323.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
