Archives of Clinical Gastroenterology
Primary retroperitoneal paraganglioma mimicking gastric GIST: Case report
Abdelilah El-Bakouri, Kabira Falousse*, Siham Elkinani, Mounir Bouali, Khalid El-Hattabi, Fatima Zahra Bensardi and Abdelaziz Fadil
Cite this as
El-Bakouri A, Falousse K, Elkinani S, Bouali M, El-Hattabi K, et al. (2024) Primary retroperitoneal paraganglioma mimicking gastric GIST: Case report. Arch Clin Gastroenterol 10(2): 007-009. DOI: 10.17352/2455-2283.000121Copyright License
© 2024 El-Bakouri A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and r eproduction in any medium, provided the original author and source are credited.Introduction: A paraganglioma is a tumor originating from the sympathetic or parasympathetic nervous system. Its diagnosis is sometimes difficult, especially if it occurs in an atypical location. We report the case of a patient who presented with a diagnosis of gastric GIST and underwent surgical resection and whose postoperative histopathological examination revealed that it was a paraganglioma.
Case report: A 38-year-old female patient who underwent cesarean section one year ago, had isolated epigastralgia for two years, the clinical examination was without abnormalities, and the patient benefited from an oesogastroduodenal fibroscopy showing an extrinsic compression aspect at the level of the greater curvature, the abdominal CT scan showed an 8 cm intraperitoneal mass in contact with the lesser gastric curvature evoking a gastrointestinal stromal tumor aspect. The biological work-up was without abnormalities. Surgery was performed to remove the retroperitoneal mass and a final diagnosis of paraganglioma was made, eliminating the initial diagnosis of gastrointestinal stromal tumor.
Discussion: There are lessons to be learned from our case first, paraganglioma can be misdiagnosed. Second, misdiagnosis can be misled by clinical diagnosis and imaging. Third, misdiagnosis can lead to high morbidities and mortality.
Conclusion: The correct knowledge of this pathology, including the differential diagnosis, is fundamental to establishing the correct diagnosis. In addition, close follow-up of these cases should be considered because of the high risk of metastatic disease.
Introduction
Paragangliomas (PGLs) are rare tumors with an annual incidence of 2-8 per million [1]. Extra-adrenal PGLs arise from chromaffin cells of the autonomic nervous system [2]. Sympathetic PGLs arise from the sympathetic chain in the thorax or retroperitoneum and parasympathetic PGLs are found in the head and neck in association with the cranial nerves [3-7]. PGLs do not often present with typical clinical symptoms and do not have unique radiological features. Their diagnosis is sometimes difficult and they are easily confused with other neoplasms, such as gastrointestinal duodenal stromal tumors (gastrointestinal stromal tumors), retroperitoneal sarcomas, neurofibromas, pancreatic neoplasms, and lymphomas [5].
Case report
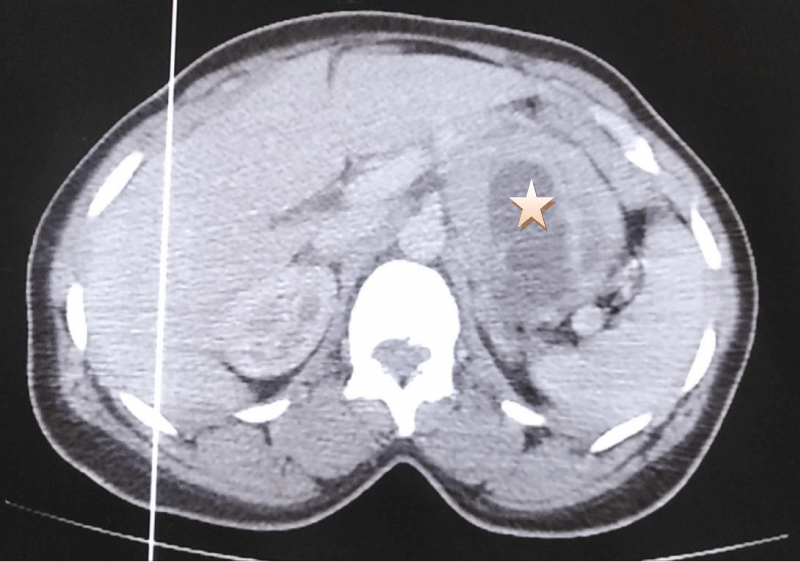
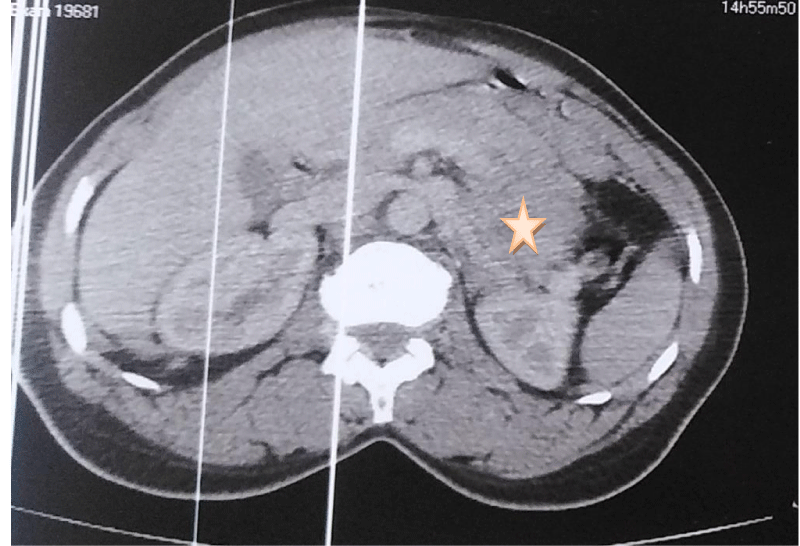
A 38-year-old patient who underwent cesarean section one year ago has had epigastralgia for two years without vomiting, transit disorders, or digestive hemorrhages, evolving in a context of apyrexia and conservation of the general state, the clinical examination found a conscious patient, PS:0, blood pressure at 130/70, heart rate at 76 bpm, respiratory rate at 20 cpm, and a normal temperature, abdominal examination: the abdomen was soft, Pfannenstiel scar, hernia of the white line, rectal examination without abnormalities, the patient benefited from an oesogastroduodenal fibroscopy objectifying an aspect of gastric obstruction at the level of the great curvature, abdominal CT: a large, well-limited, round mass, heterodense, heterogeneously enhancing after injection of PDC, delimiting central necrosis zones and measuring 8x6x5 cm, in contact with the small gastric curvature, it pushes down the pancreas and the splenic vein and comes into contact with the left surrenal. Biological findings: Hb: 11.6 g/dl, WBC: 5460/mm, CRP: 1 mg/l, ACE: 1.34 ng/ml, CA 19.9: 9.3 U/ml (Figures 1,2).
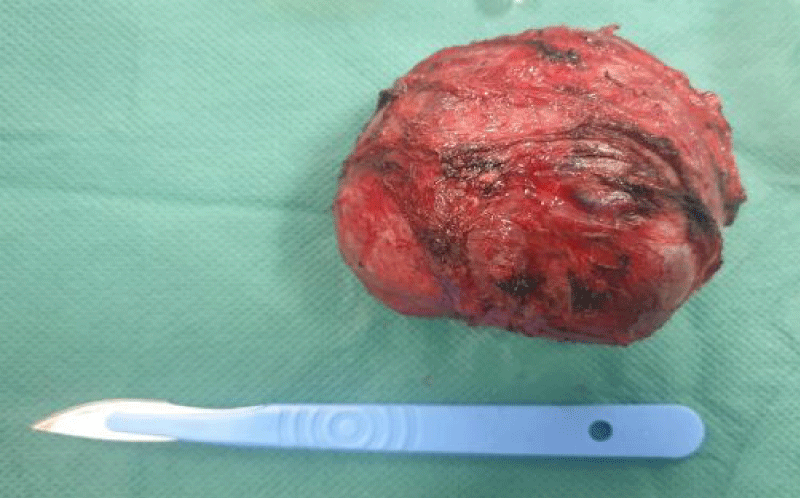
After optimization of his general condition, it was decided to perform a laparotomy, under general anesthesia with endotracheal intubation. Preoperative prophylactic antibiotics were administrated., the surgical exploration revealed the presence of a solid cystic retroperitoneal mass 10 cm in diameter (Figure 3), adherent to the superior border of the pancreas without invading it, adherent to the celiac trunk medially and to the adrenal gland laterally, with intimate contact with the abdominal aorta, without peritoneal effusion, hepatic metastases or nodules of carcinosis. The procedure involved careful dissection of the mass concerning the vascular structure using Metzenbeum forceps and bipolar forceps, with vascular control using wire and bipolar forceps, followed by resection of the mass.
The immunohistochemical study of the surgical specimen showed that the tumor cells diffusely expressed chromogranin A and that S100 was expressed by the supratentorial cells, these findings were consistent with an extra-adrenal paraganglioma.
The postoperative course was uneventful, with an improvement in the patient’s general condition. He received proton pump inhibitors (OMEPRAZOL 40 mg, one injection/day) and prophylactic heparin therapy (ENOXAPARIN SODIUM 0.4 ml = 40 mg, one injection/day) and left the hospital four days later.
The procedure was performed in the operating room of the digestive surgery department by an assistant professor of general surgery and two residents of the same specialty. The patient was satisfied with the procedure and the improvement of her health in the short and long term.
Discussion
The World Health Organization defines paraganglioma as a non-epithelial neuroendocrine tumor arising in extra-adrenal PGLs originating from the autonomic nervous system [8,9]. Sympathetic PGLs are most often located in the retroperitoneum and thorax [5].
Non-functional retroperitoneal forms are most rare and isolated [10]. Because of their location, non-secretory nature, and slow growth, they are clinically latent with normal catecholamine levels in urine and blood [11,12]. In some cases, they may manifest as symptoms of mass effect (3,13,14). The age of patients at diagnosis is usually around 40 years, with a sex ratio close to 1.3 [11,12]. Functional retroperitoneal forms secrete noradrenaline and normetanephrine and account for 30% - 60% of tumors. In the presence of a functional tumor, patients suffer from paroxysmal hypertension, as well as the typical triad of symptoms associated with pheochromocytomas: tachycardia, headache, and sweating [3,5,6,11]. In this case, the patient never presented with a hypertensive crisis, and therefore we did not perform a catecholamine assay in urine and blood.
Reperitoneal PGL is often misdiagnosed on imaging, especially in non-functioning cases [7]. In our case, the abdominal mass appears to have developed at the expense of the stomach and was diagnosed on imaging as a gastrointestinal stromal tumor [5]. Indeed, there is no specific CT criterion for PGL to differentiate it from other retroperitoneal pancreatic or gastric tumors or in the case of neurofibroma or sarcoma [6]. Abdominal CT usually shows a solid, round, or oval, homogeneous mass, but the center may have a cystic or necrotic appearance or a calcified appearance [15,16]. MRI shows a hypointense signal in T1, which is enhanced on the second T2 echo. However, MRI does not provide any benefit over CT in the characterization of PGL. (Histologically, PGLs are diagnosed by their highly vascular appearance, with main cells and sustentative cells arranged in clusters called zellballen. The principal cells are often positive for neuroendocrine markers (synaptophysin, NSE, chromogranin) on immunohistochemistry, while the sustentacular cells are positive for S-100 protein [16]. Surgical resection of the tumor is the main treatment of choice [5,15] because these tumors are potentially malignant. The diagnosis of malignancy can only be made retrospectively if distant metastases occur. Sites of metastasis include lymph nodes, bone, liver, and lung. Follow-up with metaiodobenzyl-guanidine scintigraphy can detect metastases or recurrences [16].
Given the difficulty of preoperative diagnosis in the absence of pathognomonic features, the final diagnosis of retroperitoneal PGL in our patient was made based on the histological features of the tumor and the immunohistochemical results of the S-100 positive sustentacular cells [3,8], and as we performed a complete resection of the mass (R0), we followed up the patient with abdominal ultrasound every three months for the first year [16].
Conclusion
Retroperitoneal PGLs are rare tumors, mostly benign and with a good prognosis, but can be locally invasive and metastatic. They may be functional or non-functional. Careful clinical evaluation and pathological diagnosis can prevent or reduce misdiagnosis of a retroperitoneal mass.
Ethical approval
As per international standards written ethical approval has been collected and preserved by the author(s).
Author contribution
This work was carried out in collaboration among all authors. All authors contributed to the conduct of this work. They also declare that they have read and approved the final version of the manuscript.
- Zeng J, Simsir A, Oweity T, Hajdu C, Cohen S, Shi Y. Peripancreatic paraganglioma mimics pancreatic/gastrointestinal neuroendocrine tumor on fine needle aspiration: Report of two cases and review of the literature. Diagn Cytopathol. 2017 Oct; 45(10):947-952. doi: 10.1002/dc.23761. Epub 2017 May 30. PMID: 28560856.
- Li P, Zhao D. A rare case of retroperitoneal paraganglioma-case report and literature review. Transl Gastroenterol Hepatol. 2016 Jul 18; 1:58. doi: 10.21037/tgh.2016.06.01. PMID: 28138625; PMCID: PMC5244753.
- Abbasi A, Wakeman KM, Pillarisetty VG. Pancreatic paraganglioma mimicking pancreatic neuroendocrine tumor. Rare Tumors. 2020 Dec 21; 12:2036361320982799. doi: 10.1177/2036361320982799. PMID: 33425308; PMCID: PMC7756035.
- Thakur A, Choudhary NS, Sarin H, Srivastava S, Puri R. Peripancreatic paraganglioma: A diagnostic dilemma resolved on endoscopic ultrasound guided fine needle aspiration cytology. Diagn Cytopathol. 2021 Nov; 49(11):1213-1216. doi: 10.1002/dc.24870. Epub 2021 Sep 7. PMID: 34491633.
- Huang Z, Liu H, Huang W, Wang H, Liu J, Wu Z. Giant retroperitoneal paraganglioma: Challenges of misdiagnosis and high surgical risks, a case report. Int J Surg Case Rep. 2021 Jul; 84:106081. doi: 10.1016/j.ijscr.2021.106081. Epub 2021 Jun 9. PMID: 34119947; PMCID: PMC8209176.
- Perrot G, Pavic M, Milou F, Crozes C, Faucompret S, Vincent E. Diagnostic difficile d'un paragangliome pancréatique [Difficult diagnosis of a pancreatic paraganglioma]. Rev Med Interne. 2007 Oct; 28(10):701-4. French. doi: 10.1016/j.revmed.2007.06.001. Epub 2007 Jun 19. PMID: 17618712.
- Lin S, Peng L, Huang S, Li Y, Xiao W. Primary pancreatic paraganglioma: a case report and literature review. World J Surg Oncol. 2016 Jan 22; 14(1):19. doi: 10.1186/s12957-016-0771-2. PMID: 26801079; PMCID: PMC4722732.
- Naito Y, Matsumura M, Horiguchi SI, Suzuki M, Kikuyama M, Seyama Y. Laparoscopic resection of a paraganglioma in the greater omentum mimicking a peripancreatic neoplasm: a case report. Clin J Gastroenterol. 2021 Oct; 14(5):1364-1370. doi: 10.1007/s12328-021-01452-0. Epub 2021 May 30. PMID: 34053005.
- Nguyen E. Nakasaki M, Thomas K Lee, Di Lu. Diagnosis of paraganglioma as a pancreatic mass: A case report. Recherche Google. [cité 16 mars 2023].
- Figueiredo González O, Rosero Ruiz GL, Gómez Pombo AI. Paraganglioma pancreático incidental [Incidental pancreatic paraganglioma]. Rev Esp Anestesiol Reanim. 2014 May; 61(5):292-3. Spanish. doi: 10.1016/j.redar.2013.06.016. Epub 2013 Sep 12. PMID: 24035540.
- Sangster G, Do D, Previgliano C, Li B, LaFrance D, Heldmann M. Primary retroperitoneal paraganglioma simulating a pancreatic mass: a case report and review of the literature. HPB Surg. 2010; 2010:645728. doi: 10.1155/2010/645728. Epub 2010 Dec 6. PMID: 21188160; PMCID: PMC3004405.
- Hasbi S, El Khader A, El Fahssi M, El Kaoui H, Sall I, Ali AA, Zentar A, Sair K. Case note: retroperitoneal nonfunctioning paraganglioma. Can J Surg. 2010 Feb; 53(1):E3-4. PMID: 20100402; PMCID: PMC2810007.
- Lanke G, Stewart JM, Lee JH. Pancreatic paraganglioma diagnosed by endoscopic ultrasound-guided fine needle aspiration: A case report and review of literature. World J Gastroenterol. 2021 Oct 7; 27(37):6322-6331. doi: 10.3748/wjg.v27.i37.6322. PMID: 34712035; PMCID: PMC8515802.
- Singhi AD, Hruban RH, Fabre M, Imura J, Schulick R, Wolfgang C, Ali SZ. Peripancreatic paraganglioma: a potential diagnostic challenge in cytopathology and surgical pathology. Am J Surg Pathol. 2011 Oct; 35(10):1498-504. doi: 10.1097/PAS.0b013e3182281767. PMID: 21921779.
- Tumuluru S, Mellnick V, Doyle M, Goyal B. Pancreatic Paraganglioma: A Case Report. Case Rep Pancreat Cancer. 2016 Dec 1; 2(1):79-83. doi: 10.1089/crpc.2016.0016. PMID: 30631823; PMCID: PMC6319695.
- Chattoraj AK, Rao UM, Sarkar N, Jakka S. Non-functional retroperitoneal paraganglioma: A case report. J Family Med Prim Care. 2019 Apr; 8(4):1497-1499. doi: 10.4103/jfmpc.jfmpc_189_19. PMID: 31143749; PMCID: PMC6510092.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
